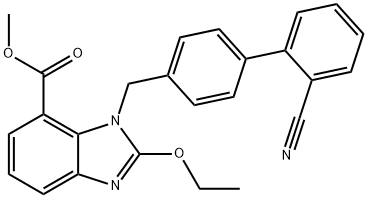
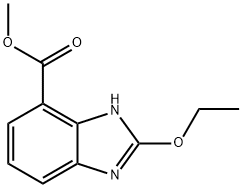
Methyl 1-[(2'-cyanobiphenyl-4-yl)methyl]-2-ethoxy-1H-benzimidazole-7-carboxylate synthesis
- Product Name:Methyl 1-[(2'-cyanobiphenyl-4-yl)methyl]-2-ethoxy-1H-benzimidazole-7-carboxylate
- CAS Number:139481-44-0
- Molecular formula:C25H21N3O3
- Molecular Weight:411.45
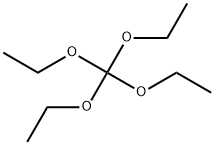
78-09-1
136304-78-4
![Methyl 1-[(2'-cyanobiphenyl-4-yl)methyl]-2-ethoxy-1H-benzimidazole-7-carboxylate](/CAS/GIF/139481-44-0.gif)
139481-44-0
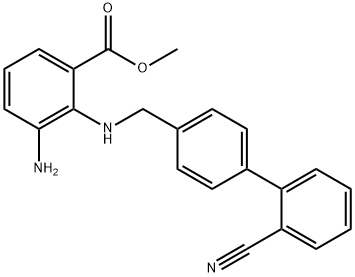
Using methyl 3-amino-2-(((2'-cyano-[1,1'-biphenyl]-4-yl)methyl)amino)benzoate (MBA) and tetraethyl orthocarbonate (TEC, 397 kg) as the raw material, the mixture was heated at reflux in the presence of acetic acid (62 kg) at 78 to 82 °C for 1 to 2 hours. After completion of the reaction, the reaction solution was cooled and methanol (1680 L), 24% aqueous sodium hydroxide (65 L) and water (2030 L) were added sequentially. The mixture was stirred at 60 to 30 °C for 2 h and the pH was adjusted with aqueous sodium hydroxide solution to 5 to 7. After cooling to below 5 °C, the precipitated crystals were separated and washed with cold water (2500 L) and cold ethyl acetate (500 L) to obtain the first batch of crystals. The mother liquor and washings were concentrated under reduced pressure, cooled to below 5 °C, and the precipitated crystals were separated and washed with cold ethyl acetate (20 L) to obtain the second batch of crystals. The first and second batches of crystals were combined, dissolved in ethyl acetate (4890 L) and heated to reflux. The crystal seeds were added at about 70 °C and then cooled to 5 °C. The crystals were separated, washed with cold ethyl acetate (200 L) and dried to give methyl 1-[(2'-cyanobiphenyl-4-yl)methyl]-2-ethoxy-1H-benzimidazole-7-carboxylate (BEC, 361 kg, 84.8% yield). The product melting point was 168.5-169.5 °C. 1H-NMR (200 MHz, CDCl3) δ: 1.42 (3H, t), 3.71 (3H, s), 4.63 (2H, q), 5.59 (2H, s), 7.09 (2H, d), 7.20 (1H, t), 7.45-7.59 (5H, m), 7.69- 7.80 (2H, m), 7.92 (1H, dd).IR (KBr) cm-1: 2225, 1725, 1550, 1480, 1430, 1280, 1250, 1040, 760, 750.

78-09-1
294 suppliers
$43.00/5mL

136304-78-4
70 suppliers
inquiry
![Methyl 1-[(2'-cyanobiphenyl-4-yl)methyl]-2-ethoxy-1H-benzimidazole-7-carboxylate](/CAS/GIF/139481-44-0.gif)
139481-44-0
332 suppliers
$9.00/5g
Yield:139481-44-0 86%
Reaction Conditions:
with acetic acid at 80; for 1 h;
Steps:
1
To a solution of compound 8 in tetraethyl orthocarbonate (5 mL), acetic acid (0.37 g) was added, and the mixture was stirred at 80 °C for 1 h, the reaction mixture was concentrated, and the residue was dissolved in ethyl acetate. Washed with water, evaporated to dryness, The crystal was recrystallized from ethyl acetate-hexane.Obtained 2.01g of colorless crystal,Yield 86%, compound 5.
References:
CN108658961,2018,A Location in patent:Paragraph 0011; 0062; 0064
150058-27-8
208 suppliers
$15.00/1g
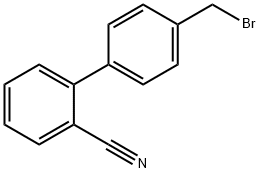
114772-54-2
624 suppliers
$5.00/5g
![Methyl 1-[(2'-cyanobiphenyl-4-yl)methyl]-2-ethoxy-1H-benzimidazole-7-carboxylate](/CAS/GIF/139481-44-0.gif)
139481-44-0
332 suppliers
$9.00/5g
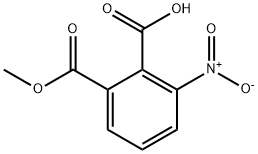
21606-04-2
148 suppliers
$7.00/1g
![Methyl 1-[(2'-cyanobiphenyl-4-yl)methyl]-2-ethoxy-1H-benzimidazole-7-carboxylate](/CAS/GIF/139481-44-0.gif)
139481-44-0
332 suppliers
$9.00/5g
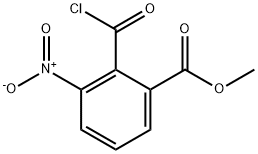
73833-13-3
8 suppliers
inquiry
![Methyl 1-[(2'-cyanobiphenyl-4-yl)methyl]-2-ethoxy-1H-benzimidazole-7-carboxylate](/CAS/GIF/139481-44-0.gif)
139481-44-0
332 suppliers
$9.00/5g
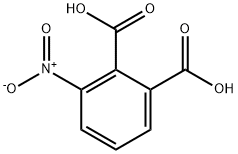
603-11-2
647 suppliers
$15.00/25g
![Methyl 1-[(2'-cyanobiphenyl-4-yl)methyl]-2-ethoxy-1H-benzimidazole-7-carboxylate](/CAS/GIF/139481-44-0.gif)
139481-44-0
332 suppliers
$9.00/5g