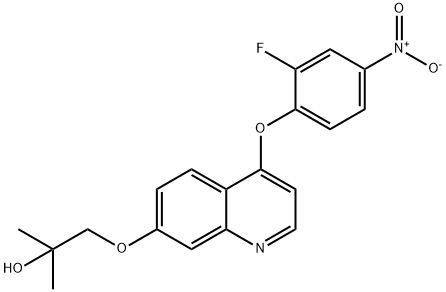
1-((4-(2-fluoro-4-nitrophenoxy)quinolin-7-yl)oxy)-2-Methylpropan-2-ol synthesis
- Product Name:1-((4-(2-fluoro-4-nitrophenoxy)quinolin-7-yl)oxy)-2-Methylpropan-2-ol
- CAS Number:1394820-99-5
- Molecular formula:C19H17FN2O5
- Molecular Weight:372.35
Yield:1394820-99-5 42.5%
Reaction Conditions:
with sodium hydroxide in tetrahydrofuran;water at 20 - 45; for 10 h;
Steps:
1.6
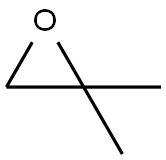
Step 6) 1-((4-(2-fluoro-4-nitrophenoxy)quinolin-7-yl)oxy)-2-methylpropan-2-ol To a solution of 4-(2-fluoro-4-nitrophenoxy)quinolin-7-ol (60 g, 0.2 mol) in THF/H2O (1 L, THF/H2O=1:1, v/v) was added NaOH (24 g, 0.6 mol) at room temperature, followed by isobutylene oxide (144 g, 2 mol). The reaction was stirred at 45° C. for 10 hours, and then diluted with EtOAc (1 L). The resulted solution was washed with 1 M NaOH aqueous solution (500 mL*4). The organic layer was separated, dried over Na2SO4 and concentrated in vacuo. The residue was washed with 500 mL of petroleum ether, and collected through filtration to give the title compound as a light yellow solid (31.6 g, 42.5%). MS (ESI, pos. ion) m/z: 373.1 [M+1]; 1H NMR (400 MHz, CDCl3): δ 1.41 (s, 6H), 2.28 (s, 1H), 3.98 (s, 2H), 6.53-6.54 (d, J=5.2 Hz, 1H), 7.26-7.36 (m, 2H), 7.45-7.46 (d, J=2.4 Hz, 1H), 8.12-8.20 (m, 3H), 8.69-8.70 (d, J=4.8 Hz, 1H).
References:
US2012/219522,2012,A1 Location in patent:Page/Page column 21
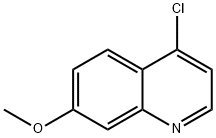
68500-37-8
194 suppliers
$18.00/250mg

1394820-99-5
9 suppliers
inquiry
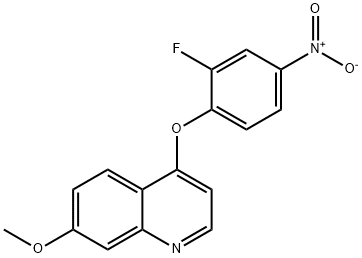
1039046-51-9
0 suppliers
inquiry

1394820-99-5
9 suppliers
inquiry
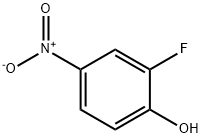
403-19-0
274 suppliers
$6.00/5g

1394820-99-5
9 suppliers
inquiry
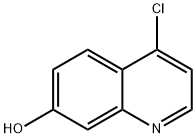
181950-57-2
93 suppliers
$80.00/0.25g

1394820-99-5
9 suppliers
inquiry