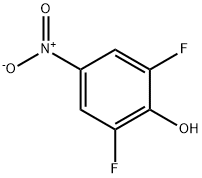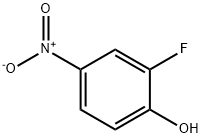
2-Fluoro-4-nitrophenol synthesis
- Product Name:2-Fluoro-4-nitrophenol
- CAS Number:403-19-0
- Molecular formula:C6H4FNO3
- Molecular Weight:157.1
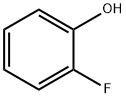
367-12-4
7440-44-0
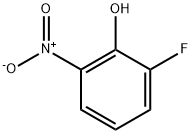
1526-17-6

403-19-0
A. Synthesis of 2-fluoro-4-nitrophenol 2-Fluorophenol (32.3 g, 0.288 mol) was dissolved in dichloromethane under ice-salt bath conditions and cooled to -10 °C. 90% nitric acid (22 g, 0.31 mol HNO3) was added slowly, and the titration rate was controlled to maintain the reaction temperature at about -5 °C, and the titration process lasted for 1 hour. After completion of the dropwise addition, the reaction mixture was continued to be stirred at 0°C for 1 hour. At the end of the reaction, the precipitate was collected by filtration and washed several times with cold dichloromethane. Analysis by GC and thin layer chromatography (silica gel, 7:3 hexane-acetone) showed that the product was mainly a single compound and the by-products generated in the reaction were of low polarity and volatility. The solid was dissolved in ether, washed with water, dried over anhydrous magnesium sulfate, and the solvent evaporated. The resulting solid was recrystallized with methylcyclohexane to give 13 g of a pale yellow solid with a melting point of 119°-121°C. Nuclear magnetic resonance (CDCl3) analysis confirmed that the product was 2-fluoro-4-nitrophenol. B. Isolation of 2-fluoro-6-nitrophenol The dichloromethane mother liquor was washed with water, dried over anhydrous magnesium sulfate and the solvent was evaporated. The resulting solid was ground with boiling hexane (3 x 150 ml) to effectively remove the least polar and volatile by-products. The hexane solution was treated with activated carbon, filtered and concentrated to about 300 ml, and cooled to give 13.5 g (30% yield) of 2-fluoro-6-nitrophenol as a yellow solid with a melting point of 70°-86° C. The solid was then dried with water.
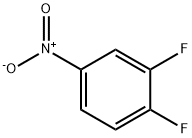
369-34-6
417 suppliers
$5.00/5g

403-19-0
272 suppliers
$6.00/5g
Yield:-
Reaction Conditions:
with hydrogenchloride in (2S)-N-methyl-1-phenylpropan-2-amine hydrate;dimethyl sulfoxide
Steps:
9.A EXAMPLE 9
(A) 57% Sodium hydride (33.7 g.) was dissolved in one liter of dimethyl sulfoxide at 70° C. When a clear solution resulted there was added slowly, with stirring, aldoxime (97.4 g.) and to the resulting pasty mixture was added slowly, with vigorous stirring, 3,4-difluoronitrobenzene (63.6 g.) and stirring was continued two hours at 35°-40° C. The reaction mixture was poured into ice water containing 80 ml. concentrated hydrochloric acid, extracted with ether and the ether solution was extracted with dilute sodium hydroxide. The alkaline extract was acidified, extracted with ether, and the ether was evaporated to give 44.5 g. of crystalline 3-fluoro-4-hydroxynitrobenzene.
References:
Sterling Drug Inc. US4224335, 1980, A
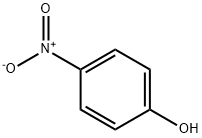
100-02-7
61 suppliers
$11.00/5G

403-19-0
272 suppliers
$6.00/5g
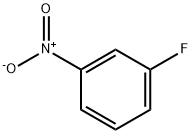
402-67-5
273 suppliers
$6.00/5g

403-19-0
272 suppliers
$6.00/5g

367-12-4
425 suppliers
$5.00/10g
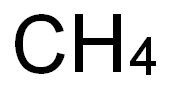
7440-44-0
354 suppliers
$12.00/25g

1526-17-6
191 suppliers
$6.00/1g

403-19-0
272 suppliers
$6.00/5g