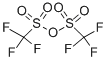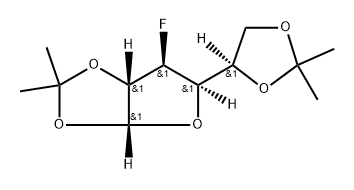
(3aR,6S,6aS)-5-((S)-2,2-dimethyl-1,3-dioxolan-4-yl)-6-fluoro-2,2-dimethyltetrahydrofuro[2,3-d][1,3]dioxole synthesis
- Product Name:(3aR,6S,6aS)-5-((S)-2,2-dimethyl-1,3-dioxolan-4-yl)-6-fluoro-2,2-dimethyltetrahydrofuro[2,3-d][1,3]dioxole
- CAS Number:14049-05-9
- Molecular formula:C12H19FO5
- Molecular Weight:262.28
2595-05-3
250 suppliers
$7.00/1g
![(3aR,6S,6aS)-5-((S)-2,2-dimethyl-1,3-dioxolan-4-yl)-6-fluoro-2,2-dimethyltetrahydrofuro[2,3-d][1,3]dioxole](/CAS/20211123/GIF/14049-05-9.gif)
14049-05-9
16 suppliers
inquiry
Yield:14049-05-9 95%
Reaction Conditions:
with pyridine in dichloromethane;toluene at -78 - 40; for 168 h;
Steps:
41.1 (3aR.5R.6S.6aS)-5-r(4R)-2.2-dimethyl-1.3-dioxolan-4-yll-6-fluoro-2.2-dimethyl-3a.5.6.6a- tetrahy drofuro [2.3 -dl [1.31 dioxole
[00910] Allofuranose diacetonide (10 g, 38.4 mmol) was dissolved in 35 mL of dry DCM and pyridine at -78 °C. To the cold solution, 2.7 M Deoxyfluor in toluene (20 mL, 54 mmol) was added dropwise. The reaction was stirred for 7 days at 40 °C. The reaction was quenched with sat NaHCCb, and the aqueous phase was extracted with DCM (3 x 50 mL). Combined organic phases were washed with NaHCC (100 mL) and dried with Na2S04. The crude material was purified by MPLC to afford the title compound (9.54 g, 36.4 mmol, 95%). Acetonides fragment on mass spec: MS (ESI) [M - both acetonides + Na]+ = 205.1.
References:
WO2019/46126,2019,A1 Location in patent:Paragraph 00910
14686-89-6
128 suppliers
$16.00/100mg
![(3aR,6S,6aS)-5-((S)-2,2-dimethyl-1,3-dioxolan-4-yl)-6-fluoro-2,2-dimethyltetrahydrofuro[2,3-d][1,3]dioxole](/CAS/20211123/GIF/14049-05-9.gif)
14049-05-9
16 suppliers
inquiry

208525-43-3
0 suppliers
inquiry
![(3aR,6S,6aS)-5-((S)-2,2-dimethyl-1,3-dioxolan-4-yl)-6-fluoro-2,2-dimethyltetrahydrofuro[2,3-d][1,3]dioxole](/CAS/20211123/GIF/14049-05-9.gif)
14049-05-9
16 suppliers
inquiry

55951-90-1
0 suppliers
inquiry
![(3aR,6S,6aS)-5-((S)-2,2-dimethyl-1,3-dioxolan-4-yl)-6-fluoro-2,2-dimethyltetrahydrofuro[2,3-d][1,3]dioxole](/CAS/20211123/GIF/14049-05-9.gif)
14049-05-9
16 suppliers
inquiry