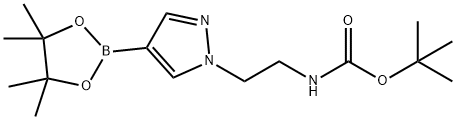
tert-butyl (2-(4-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)-1H-pyrazol-1-yl)ethyl)carbamate synthesis
- Product Name:tert-butyl (2-(4-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)-1H-pyrazol-1-yl)ethyl)carbamate
- CAS Number:1414475-01-6
- Molecular formula:C16H28BN3O4
- Molecular Weight:337.22
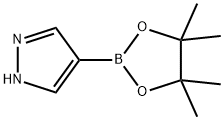
269410-08-4
399 suppliers
$5.00/1g
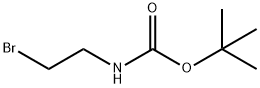
39684-80-5
255 suppliers
$17.30/250mg

1414475-01-6
16 suppliers
inquiry
Yield:1414475-01-6 35%
Reaction Conditions:
with caesium carbonate in acetonitrile at 60; for 12 h;
Steps:
168.1 tert-butyl (2-(4-(4,4,5,5-tetramethyl-I ,3,2-dioxaborolan-2-yl)-1H-pyrazol-1-yl)ethyl)earbamate
To a solution of 4-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)-IH-pyrazole (2 g, 103 mol)in MeCN (30 mL) was added tert-butyl (2-bromoethyl)carbamate (3.46 g, 15.5 mmol) andCs2CO3 (10.1 g, 30.9 mmol), The mixture was heated to 60 °C for 12 h. After cooling to It, the reaction was diluted in water (80 mL) and washed with EtOAc (80 mL x 2). The combined organic layers were dried over anhydrous Na2SO4, filtered and concentrated in vacuo. The crude product was purified by silica gel chromatography (petroleum ether/EtOAc= 8/1) to give the title compound (1.2 g, 35%) as a white solid.
References:
WO2016/86200,2016,A1 Location in patent:Page/Page column 616