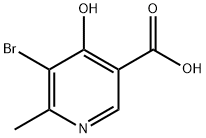
5-bromo-4-hydroxy-6-methylpyridine-3-carboxylic acid synthesis
- Product Name:5-bromo-4-hydroxy-6-methylpyridine-3-carboxylic acid
- CAS Number:141703-16-4
- Molecular formula:C7H6BrNO3
- Molecular Weight:232.03
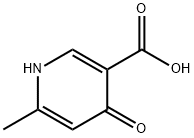
33821-58-8
34 suppliers
$46.00/1g

141703-16-4
8 suppliers
inquiry
Yield:141703-16-4 92%
Reaction Conditions:
with bromine;acetic acid at 20; for 42 h;
Steps:
1.1a 5-Bromo-6-methyl-4-oxo- 1 ,4-dihydro-pyridine-3-carboxylic acid
To a solution of 4-hydroxy-6-methyl-nicotinic acid (10.00 g, 65.3 mmol) in glacial acetic acid (35 mL) is added bromine (4.00 mL, 78.1 mmol). After stirring for 18 h at room temperature, additional bromine (0.5 mL) is added and the reaction mixture is stirred for an additional 24 h. The reaction mixture is evaporated under reduced pressure and the remaining residue is co-evaporated with toluene. The remaining residue is treated with a small amount of MeOH and then triturated with water. The precipitate is filtered off and dried. Yield: 13.8 g (92% of theory); ESI mass spectrum: [M+H]+ = 232 (bromine isotope pattern); Retention time HPLC: 0.61 min (Z002 002)
References:
WO2014/29832,2014,A1 Location in patent:Page/Page column 34
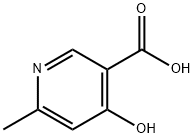
67367-33-3
207 suppliers
$6.00/5g

141703-16-4
8 suppliers
inquiry