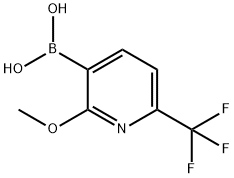
(2-Methoxy-6-(trifluoroMethyl)pyridin-3-yl)boronic acid synthesis
- Product Name:(2-Methoxy-6-(trifluoroMethyl)pyridin-3-yl)boronic acid
- CAS Number:1429874-11-2
- Molecular formula:C7H7BF3NO3
- Molecular Weight:220.94
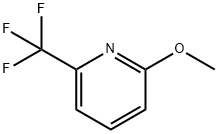
34486-18-5
65 suppliers
$145.00/1g
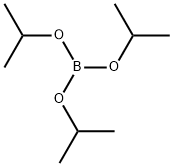
5419-55-6
386 suppliers
$12.00/5g

1429874-11-2
31 suppliers
inquiry
Yield:1429874-11-2 59%
Reaction Conditions:
Stage #1:2-methoxy-6-(trifluoromethyl)pyridine with n-butyllithium in diethyl ether;hexane at -78 - 20; for 0.583333 h;Inert atmosphere;
Stage #2:Triisopropyl borate in diethyl ether;hexane at -78 - 20; for 0.75 h;
Stage #3: with hydrogenchloride in diethyl ether;hexane;water
Steps:
16 2-methoxy-6-f trifluoromethyl)-3-pyridyll boronic acid
2-methoxy-6-f trifluoromethyl)-3-pyridyll boronic acid To a solution of 2-methoxy-6-(trifluoromethyl)pyridine (1.0 g, 5.6 mmol) and in diethyl ether (1.2 mL/mmol, 6.8 mL) at -78 C under nitrogen was added nBuLi (2.5 mol/L) in hexanes (4.7 g, 17 mmol, 6.8 mL) over 5 min and allowed to warm up to room temperature over 30 minutes. Boric acid triisopropyl ester (2.1 g, 11 mmol, 2.6 mL) in diethyl ether (1.2 mL/mmol, 6.8 mL) was cooled to -78 C and [2-methoxy-6-(trifluoromethyl)-3-pyridyl]lithium was added to this solution over 15 minutes and then warmed up to room temperature over 30 mins. Hydrogen chloride (aqueous 25%>) (10 mL, 10 mmol) was added and the reaction mixture diluted with water and extracted twice with dichloromethane, passed through a phase separator and reduced under vacuum to give a yellow oil which solidified overnight. The reaction mixture was adsorbed onto silica and purified by chromatography on silica, eluting with 0-25% ethyl acetate/hexane. Fractions containing product were combined to give [2-methoxy-6-(trifluoromethyl)-3-pyridyl]boronic acid as a yellow solid (707 mg, 3.20 mmol, 59% yield). IHNMR (llvu941hl, CDC13): δ 8.29 (d, J=7.5 Hz, 1 H) 7.34 (d, J=7.5 Hz, 1 H) 5.92 (s, 2 H) 4.10 (s, 3 H);
References:
SYNGENTA LIMITED;BHONOAH, Yunas;ELLIOTT, Alison Clare;GAULIER, Steven;LING, Kenneth;MITCHELL, Glynn;MORRIS, James Alan;RZEPA, Paula Rocha;VINER, Russell Colin WO2013/50421, 2013, A1 Location in patent:Page/Page column 59; 60
34486-06-1
258 suppliers
$12.00/1g

1429874-11-2
31 suppliers
inquiry