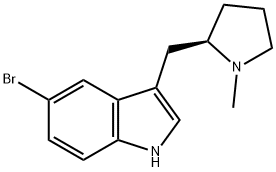
(R)-5-Bromo-3-((1-methylpyrrolidin-2-yl)methyl)-1H-indole synthesis
- Product Name:(R)-5-Bromo-3-((1-methylpyrrolidin-2-yl)methyl)-1H-indole
- CAS Number:143322-57-0
- Molecular formula:C14H17BrN2
- Molecular Weight:293.2
![1H-Indole, 5-bromo-3-[[(2R)-1-methyl-2-pyrrolidinyl]methyl]-, ethanedioate (1:1)](/CAS/20211123/GIF/1196663-29-2.gif)
1196663-29-2

143322-57-0
The general procedure for the synthesis of (R)-5-bromo-3-(1-methyl-2-pyrrolidinylmethyl)-1H-indole from the compound (CAS:1196663-29-2) is as follows: Example 26; Preparation of BIP from BIP oxalate salt BIP oxalate (170 g, 0.4435 mol) and deionized water (1500 mL) were stirred at 15 °C. The pH was adjusted to 7.8 using aqueous sodium carbonate (70.5 g, 0.6650 mol, dissolved in 200 mL of deionized water.) Toluene (1000 mL) was added and stirred for 30 min at 30-35°C. The organic and aqueous layers were separated and the aqueous layer was extracted with toluene (500 mL). Separate the organic and aqueous layers again. The organic layer was combined and washed with 10% (w/v) sodium carbonate aqueous solution. After separating the organic and aqueous layers, the organic layer was concentrated to 70% of the original volume (~1050 mL) at 50-55°C under reduced pressure. The concentrate was gradually cooled to 5-10 °C with stirring to precipitate a solid product. The solid was collected by filtration and washed sequentially with cold toluene (100 mL) and n-heptane (100 mL). After drying, 117.0 g of product was obtained; yield: 90.5%; purity: 99.12% (as BIP).
![1H-Indole, 5-bromo-3-[[(2R)-1-methyl-2-pyrrolidinyl]methyl]-, ethanedioate (1:1)](/CAS/20211123/GIF/1196663-29-2.gif)
1196663-29-2
1 suppliers
inquiry

143322-57-0
165 suppliers
$18.00/1g
Yield:143322-57-0 90.5%
Reaction Conditions:
with sodium carbonate in water;toluene at 30 - 35; pH=7.8; for 0.5 h;
Steps:
26
Example 26; Preparation of BIP from BIP OxalateBIP oxalate salt (170 g, 0.4435 mol) and DM water (1500 mL) was stirred at 15° C. The pH was adjusted to 7.8 using aqueous sodium carbonate (70.5 g, 0.6650 mol) in 200 mL DM water. Toluene (1000 mL) was added and stirred at 30-35° C. for 30 minutes. The layers were separated and the aqueous layer was washed with toluene (500 mL). The layers were again separated. The two toluene layer were combined and washed with 10% w/v aqueous sodium carbonate. The layers were separated and the toluene layer was distilled under reduce pressure at 50-55° C. to 70% (1050 mL). The distilled mass was gradually cooled to 5-10° C. with stirring. A solid product precipitated and was collected by filtration and washed, first with cold toluene (100 mL) and then with n-heptane (100 mL). Dry. Wt: 117.0 g; Yield: 90.5%; Purity: 99.12% (as a Bip).
References:
US2009/299077,2009,A1 Location in patent:Page/Page column 9-10
![(R)-5-BROMO-3-[(1-METHYL-2-PYRROLIDINYL)METHYL]-1H-INDOLE](/CAS/GIF/143322-56-9.gif)
143322-56-9
65 suppliers
$125.09/10mg

143322-57-0
165 suppliers
$18.00/1g
![1-Pyrrolidinecarboxylic acid, 2-[(5-bromo-1H-indol-3-yl)carbonyl]-, phenyl ester, (2R)-](/CAS/20210305/GIF/1353991-68-0.gif)
1353991-68-0
0 suppliers
inquiry

143322-57-0
165 suppliers
$18.00/1g
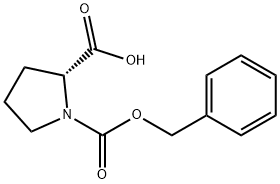
6404-31-5
369 suppliers
$8.00/1g

143322-57-0
165 suppliers
$18.00/1g
![(R)-N-[(phenylmethoxy)carbonyl]-2-pyrrolidinecarbonyl chloride](/CAS/GIF/61350-62-7.gif)
61350-62-7
8 suppliers
inquiry

143322-57-0
165 suppliers
$18.00/1g