(R)-5-BROMO-3-[(1-METHYL-2-PYRROLIDINYL)METHYL]-1H-INDOLE synthesis
- Product Name:(R)-5-BROMO-3-[(1-METHYL-2-PYRROLIDINYL)METHYL]-1H-INDOLE
- CAS Number:143322-56-9
- Molecular formula:C21H19BrN2O3
- Molecular Weight:427.29
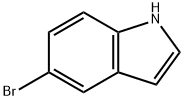
10075-50-0
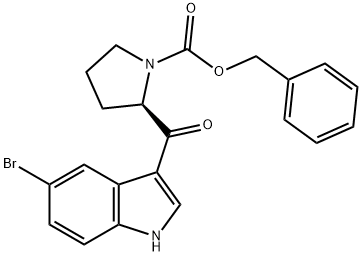
![(R)-N-[(phenylmethoxy)carbonyl]-2-pyrrolidinecarbonyl chloride](/CAS/GIF/61350-62-7.gif)
61350-62-7
![(R)-5-BROMO-3-[(1-METHYL-2-PYRROLIDINYL)METHYL]-1H-INDOLE](/CAS/GIF/143322-56-9.gif)
143322-56-9
General procedure for the synthesis of benzyl (R)-2-(5-bromo-1H-indole-3-carbonyl)pyrrolidine-1-carboxylate from 5-bromoindole and benzyl (R)-2-(chlorocarbonyl)pyrrolidine-1-carboxylate: 5-bromoindole (15.7 g, 80.4 mmol) was added to a 500 mL four-necked flask with dichloromethane (140 mL). The temperature of the reaction system was lowered to 0 °C under cooling in an ice bath, and aluminum trichloride solid (16.1 g, 120.6 mmol) was slowly added, taking care to add it in batches and controlling the internal temperature between 0 and 5 °C. After addition, the reaction was continued to be stirred at 0~5°C for 1 hour. Subsequently, a dichloromethane solution of N-benzyloxycarbonyl prolyl chloride prepared in Example 1 was slowly added dropwise under the same temperature conditions. After the dropwise addition was completed, the reaction was continued to be stirred for 6 hours by keeping 0~5°C. At the end of the reaction, the reaction system was placed in an ice bath and the reaction was quenched by slowly dropping 90 mL of saturated ammonium chloride solution. The reaction mixture was filtered, the filtrate was separated and the organic phase was washed with saturated sodium bicarbonate solution and saturated brine sequentially. After the solvent was removed by concentration under reduced pressure, the resulting crude product was purified by recrystallization using a solvent mixture of ethyl acetate and n-heptane (1:1, v/v). The purified product was dried under vacuum at 60 °C for 8 h to afford (R)-3-(N-benzyloxycarbonylpyrrolidin-2-ylcarbonyl)-5-bromo-1H-indole (14.2 g) as a white solid in 83% yield and 99.2% HPLC purity.

10075-50-0
689 suppliers
$5.00/10g
![(R)-N-[(phenylmethoxy)carbonyl]-2-pyrrolidinecarbonyl chloride](/CAS/GIF/61350-62-7.gif)
61350-62-7
8 suppliers
inquiry
![(R)-5-BROMO-3-[(1-METHYL-2-PYRROLIDINYL)METHYL]-1H-INDOLE](/CAS/GIF/143322-56-9.gif)
143322-56-9
65 suppliers
$125.09/10 mg
Yield:143322-56-9 83%
Reaction Conditions:
Stage #1:5-bromo-1H-indole with aluminum (III) chloride in dichloromethane at 0 - 5; for 1 h;
Stage #2:N-benzyloxycarbonyl-D-proline acid chloride in dichloromethane at 0 - 5; for 6 h;Reagent/catalyst;Concentration;Temperature;Solvent;
Steps:
4 Example 4
5-bromoindole (15.7 g, 80.4 mmol), dichloromethane (140 mL) was added to a 500 ml four-necked flask,The ice bath was cooled to 0 ° C and the aluminum trichloride solid (16.1 g, 120.6 mmol) was slowly added in portions,The internal temperature control in the 0 ~ 5 , stirring reaction 1h, 0 ~ 5 conditions,A solution of N-benzyloxycarbonyl prolyloyl chloride in Example 1 in dichloromethane was added dropwise,The reaction solution was stirred at 0-5 ° C for 6 h. Ice bath, 90mL saturated ammonium chloride solution slowly drip,First filter, the filtrate layer, the organic phase in turn with saturated sodium bicarbonate, saturated salt water washing,After concentration under reduced pressure, the crude product was purified by recrystallization from a mixed solvent of ethyl acetate and n-heptane (1: 1 by volume), vacuum dried at 60 ° C for 8 hours,(R) -3- (N-benzyloxycarbonylpyrrolidin-2-ylcarbonyl) -5-bromo-lH-indole (14.2 g) as a white solid,The yield was 83% and the HPLC purity was 99.2%.
References:
Shanghai Pharmaceutical Industry Research Institute;China Medical Industry Research Institute;Sun, Hui;Zhou, Weicheng;Zhang, Shunli CN104230895, 2017, B Location in patent:Paragraph 0031; 0032; 0033; 0034; 0035; 0036-0044

10075-50-0
689 suppliers
$5.00/10g

144-55-8
1410 suppliers
$10.00/25g
![(R)-N-[(phenylmethoxy)carbonyl]-2-pyrrolidinecarbonyl chloride](/CAS/GIF/61350-62-7.gif)
61350-62-7
8 suppliers
inquiry
![(R)-5-BROMO-3-[(1-METHYL-2-PYRROLIDINYL)METHYL]-1H-INDOLE](/CAS/GIF/143322-56-9.gif)
143322-56-9
65 suppliers
$125.09/10 mg

10075-50-0
689 suppliers
$5.00/10g
676-58-4
292 suppliers
$15.00/25ml
![(R)-N-[(phenylmethoxy)carbonyl]-2-pyrrolidinecarbonyl chloride](/CAS/GIF/61350-62-7.gif)
61350-62-7
8 suppliers
inquiry
![(R)-5-BROMO-3-[(1-METHYL-2-PYRROLIDINYL)METHYL]-1H-INDOLE](/CAS/GIF/143322-56-9.gif)
143322-56-9
65 suppliers
$125.09/10 mg

10075-50-0
689 suppliers
$5.00/10g

79-37-8
490 suppliers
$17.67/10gm:
925-90-6
348 suppliers
$12.00/5ml
![(R)-N-[(phenylmethoxy)carbonyl]-2-pyrrolidinecarbonyl chloride](/CAS/GIF/61350-62-7.gif)
61350-62-7
8 suppliers
inquiry
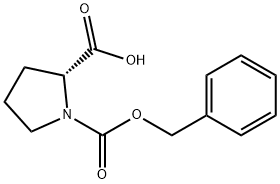
6404-31-5
369 suppliers
$8.00/1g
![(R)-5-BROMO-3-[(1-METHYL-2-PYRROLIDINYL)METHYL]-1H-INDOLE](/CAS/GIF/143322-56-9.gif)
143322-56-9
65 suppliers
$125.09/10 mg

6404-31-5
369 suppliers
$8.00/1g
![(R)-5-BROMO-3-[(1-METHYL-2-PYRROLIDINYL)METHYL]-1H-INDOLE](/CAS/GIF/143322-56-9.gif)
143322-56-9
65 suppliers
$125.09/10 mg