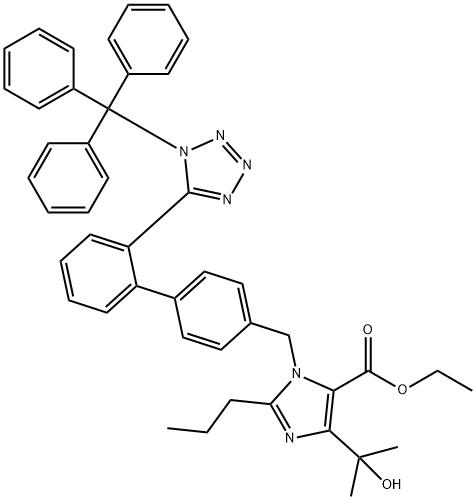
1H-IMidazole-5-carboxylic acid, 4-(1-hydroxy-1-Methylethyl)-2-propyl-1-[[2'-[1-(triphenylMethyl)-1H-tetrazol-5-yl][1,1'-biphenyl]-4-yl]Methyl]-, ethyl ester synthesis
- Product Name:1H-IMidazole-5-carboxylic acid, 4-(1-hydroxy-1-Methylethyl)-2-propyl-1-[[2'-[1-(triphenylMethyl)-1H-tetrazol-5-yl][1,1'-biphenyl]-4-yl]Methyl]-, ethyl ester
- CAS Number:144690-33-5
- Molecular formula:C45H44N6O3
- Molecular Weight:716.87
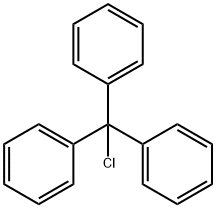
76-83-5
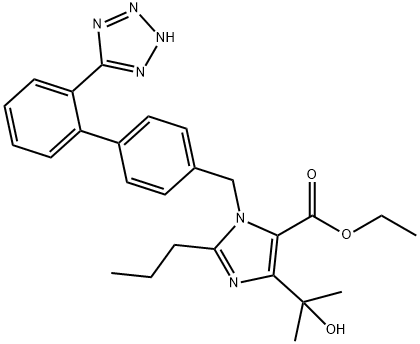
144689-23-6
![1H-IMidazole-5-carboxylic acid, 4-(1-hydroxy-1-Methylethyl)-2-propyl-1-[[2'-[1-(triphenylMethyl)-1H-tetrazol-5-yl][1,1'-biphenyl]-4-yl]Methyl]-, ethyl ester](/CAS/GIF/144690-33-5.gif)
144690-33-5
To ethyl 4-(1-hydroxy-1-methylethyl)-2-propyl-1-[4-[2-(tetrazol-5-yl)phenyl]phenyl]methylimidazole-5-carboxylate (BIH, 100 g, 1 eq.) was added dichloromethane (500 mL, 5 vol.), and stirred until completely dissolved. Triethylamine (32.3 mL, 1.1 eq.) was added and the reaction mixture was cooled to 0 °C - 5 °C. A solution of triphenylchloromethane (63.22 g, 1.08 eq.) in dichloromethane (300 mL, 3 vol.) was slowly added dropwise over 30 min at 0 °C-5 °C. After the dropwise addition was completed, the reaction mixture was warmed to 25°C-30°C with continuous stirring for 12 hours. Triphenylchloromethane (2.92 g, 0.05 eq.) was added additionally and stirring was continued for 3 hours. The reaction was monitored by thin layer chromatography (TLC, unfolding agent: 10% methanol/dichloromethane, UV detection) to confirm complete conversion of BIH. The reaction mixture was cooled to 0°C-5°C, demineralized water (270 mL, 2.7 v/v) was added, and stirred at 25°C-30°C for 15 min. Layers were left to separate and the aqueous phase was extracted with dichloromethane (200 mL, 2 volumes). The organic phases were combined and washed with deionized water (500 mL, 5 volumes). The organic phase was dried over anhydrous sodium sulfate, filtered, and the filtrate was concentrated under reduced pressure at 40°C-45°C to afford ethyl 4-(1-hydroxy-1-methylethyl)-2-propyl-1-[4-[2-(triphenylmethyltetrazol-5-yl)phenyl]phenyl]methylimidazole-5-carboxylate (BIT, 135 g, 89% yield).
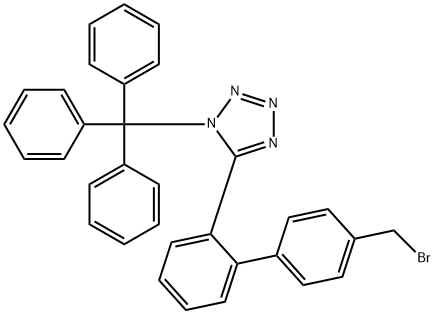
124750-51-2
464 suppliers
$6.00/1g
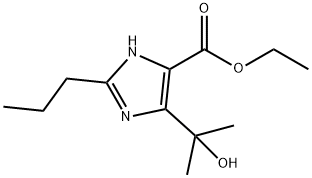
144689-93-0
491 suppliers
$16.00/5g
![1H-IMidazole-5-carboxylic acid, 4-(1-hydroxy-1-Methylethyl)-2-propyl-1-[[2'-[1-(triphenylMethyl)-1H-tetrazol-5-yl][1,1'-biphenyl]-4-yl]Methyl]-, ethyl ester](/CAS/GIF/144690-33-5.gif)
144690-33-5
138 suppliers
inquiry
Yield: 93.8%
Reaction Conditions:
Stage #1:N-(triephenylmethyl)-5-<4'-(bromomethyl)-biphenyl-2-yl>tetrazole;ethyl 4-(2-hydroxypropan-2-yl)-2-propyl-1H-imidazole-5-carboxylate with potassium carbonate in butanone at 60; for 2 h;
Stage #2: with N,N-dimethyl acetamide in butanone at 45; for 4 h;
Steps:
1 Example 1: Preparation of Compound 3
Weigh 24.0 g of compound 1, 55.7 g of compound 2, And add 124g of potassium carbonate, Then 200 mL of butanone was added, Warmed to 60 ° C, Stir for two hours, Cool to 45 , The composite catalyst (a mixture of polyethylene glycol 400 and N, N-dimethylacetamide in a mass ratio of 5: 1) Continue stirring for 4 hours, TLC detection, Show two kinds of raw materials are not left. The reaction is over, filter, The filtrate was collected, concentrate, Get oil, A mixture of 50 mL of ethanol and water (2: 1 by volume) Stirring, A large number of solid precipitation, filter, Collect the solid, Washed with 50mL n-hexane beating, The target compound 67.3g, Yield 93.8% The HPLC purity was 99.3%.
References:
Disha Pharmaceutical Group Co., Ltd.;Zhang Zhaoxing;Zhang Hongqiang;Qin Litai;Li Wei;Li Zongwen;Xia Haijian CN103012382, 2016, B Location in patent:Paragraph 0015; 0026-0027
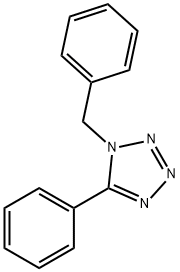
28386-90-5
15 suppliers
inquiry
![1H-IMidazole-5-carboxylic acid, 4-(1-hydroxy-1-Methylethyl)-2-propyl-1-[[2'-[1-(triphenylMethyl)-1H-tetrazol-5-yl][1,1'-biphenyl]-4-yl]Methyl]-, ethyl ester](/CAS/GIF/144690-33-5.gif)
144690-33-5
138 suppliers
inquiry
![Ethanamine, N-[(3,5-dichlorophenyl)methylene]-2,2-diethoxy-](/CAS/20210305/GIF/1000210-73-0.gif)
1000210-73-0
0 suppliers
inquiry
![1H-IMidazole-5-carboxylic acid, 4-(1-hydroxy-1-Methylethyl)-2-propyl-1-[[2'-[1-(triphenylMethyl)-1H-tetrazol-5-yl][1,1'-biphenyl]-4-yl]Methyl]-, ethyl ester](/CAS/GIF/144690-33-5.gif)
144690-33-5
138 suppliers
inquiry
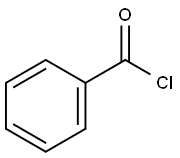
98-88-4
596 suppliers
$10.00/5g
![1H-IMidazole-5-carboxylic acid, 4-(1-hydroxy-1-Methylethyl)-2-propyl-1-[[2'-[1-(triphenylMethyl)-1H-tetrazol-5-yl][1,1'-biphenyl]-4-yl]Methyl]-, ethyl ester](/CAS/GIF/144690-33-5.gif)
144690-33-5
138 suppliers
inquiry