ethyl 4-hydroxy-5,6,7,8-tetrahydrobenzo[4,5]thieno[2,3-d]pyriMidine-6-carboxylate synthesis
- Product Name:ethyl 4-hydroxy-5,6,7,8-tetrahydrobenzo[4,5]thieno[2,3-d]pyriMidine-6-carboxylate
- CAS Number:1447956-28-6
- Molecular formula:C13H14N2O3S
- Molecular Weight:278.33
Yield:1447956-28-6 55%
Reaction Conditions:
in formamide at 180; for 4 h;Inert atmosphere;
Steps:
7.6 Synthesis of ethyl 4-hydroxy-5,6,7,8-tetrahydrobenzo[4,5]thieno[2,3- d] pyrimidine-6-carboxylate
A solution of 3,5-diethyl 2-amino-4,5,6,7-tetrahydro-l-benzothiophene-3,5- dicarboxylate (8 g, 26.90 mmol, 1.00 equiv) in formamide (150 mL) was added formamidine acetate (10 g, 96.05 mmol, 3.57 equiv) at room temperature under nitrogen. The resulting solution was stirred for 4 h at 180°C. After cooling, the resulting solution was diluted with 200 mL of EtOAc, washed with water and brine, dried over anhydrous sodium sulfate and concentrated under vacuum. The residue was applied onto a silica gel column with ethyl acetate/petroleum ether (1 :4) give the desired ethyl 4-hydroxy-5, 6,7,8- tetrahydrobenzo[4,5]thieno[2,3-d]pyrimidine-6-carboxylate (4.1 g, 55%) as a yellow solid.
References:
WO2014/11902,2014,A1 Location in patent:Paragraph 00290-00291
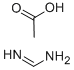
99-06-9
525 suppliers
$5.00/25g
![ethyl 4-hydroxy-5,6,7,8-tetrahydrobenzo[4,5]thieno[2,3-d]pyriMidine-6-carboxylate](/CAS/20150408/GIF/1447956-28-6.gif)
1447956-28-6
7 suppliers
inquiry
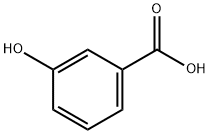
606488-94-2
65 suppliers
$45.00/100mg
![ethyl 4-hydroxy-5,6,7,8-tetrahydrobenzo[4,5]thieno[2,3-d]pyriMidine-6-carboxylate](/CAS/20150408/GIF/1447956-28-6.gif)
1447956-28-6
7 suppliers
inquiry
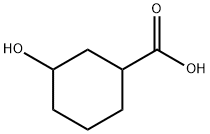
16205-98-4
107 suppliers
$45.00/25mg
![ethyl 4-hydroxy-5,6,7,8-tetrahydrobenzo[4,5]thieno[2,3-d]pyriMidine-6-carboxylate](/CAS/20150408/GIF/1447956-28-6.gif)
1447956-28-6
7 suppliers
inquiry
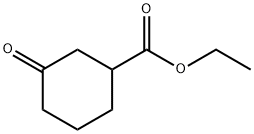
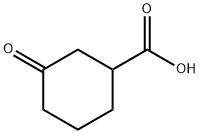
33668-25-6
141 suppliers
$24.73/50mgs:
![ethyl 4-hydroxy-5,6,7,8-tetrahydrobenzo[4,5]thieno[2,3-d]pyriMidine-6-carboxylate](/CAS/20150408/GIF/1447956-28-6.gif)
1447956-28-6
7 suppliers
inquiry
![diethyl 2-aMino-4,5,6,7-tetrahydrobenzo[b]thiophene-3,5-dicarboxylate](/CAS/20150408/GIF/1029689-49-3.gif)