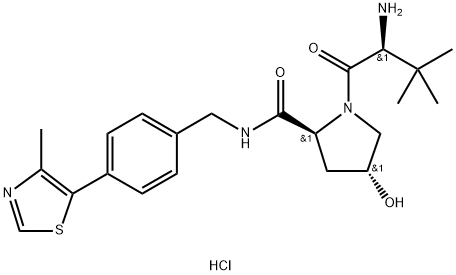
(2S,4R)-1-((S)-2-amino-3,3-dimethylbutanoyl)-4-hydroxy-N-(4-(4-methylthiazol-5-yl)benzyl)pyrrolidine-2-carboxamide hydrochloride synthesis
- Product Name:(2S,4R)-1-((S)-2-amino-3,3-dimethylbutanoyl)-4-hydroxy-N-(4-(4-methylthiazol-5-yl)benzyl)pyrrolidine-2-carboxamide hydrochloride
- CAS Number:1448189-80-7
- Molecular formula:C22H31ClN4O3S
- Molecular Weight:467.03
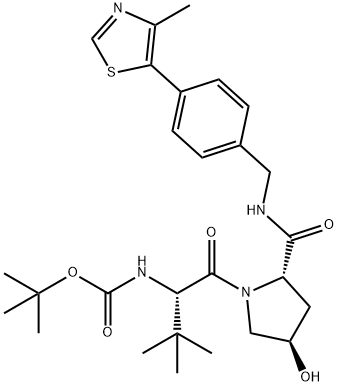
1448189-98-7

1448189-80-7
Step 4: Synthesis of (2S,4R)-1-((S)-2-amino-3,3-dimethylbutanoyl)-4-hydroxy-N-(4-(4-methylthiazol-5-yl)benzyl)pyrrolidine-2-carboxamide hydrochloride (ULM-1) To a stirred solution of tert-butyl N-[(2S)-1-[(2S,4R)-4-hydroxy-2-({[4-(4-methyl-1,3-thiazol-5-yl)phenyl]methyl}carbamoyl)pyrrolidin-1-yl]-3,3-dimethyl-1-oxobutan-2-yl]carbamate (12 g, 22.61 mmol) in dioxane (20 mL) at room temperature was added to a dioxane solution of hydrogen chloride (4N, 80 mL). The reaction mixture was stirred at room temperature for 2 h. LC-MS monitoring showed formation of the target product. The precipitated solid was collected by filtration to give ULM-1 in 48% yield as a yellow solid. 1H NMR (400 MHz, CD3OD): δ 9.84-9.82 (s, 1H), 7.58-7.54 (m, 4H), 4.71-4.41 (m, 4H), 4.13-4.08 (m, 1H), 3.86-3.71 (m, 2H), 3.36 (s, 1H), 2.60-2.58 (s, 3H), 2.35-2.07 (m, 2H), 1.19-1.12 (m, 9H). LC-MS (ES+): m/z 431.11 [MH+], tR = 0.73 min (run time 2.0 min).

1448189-98-7
48 suppliers
inquiry

1448189-80-7
141 suppliers
$20.00/5mg
Yield:1448189-80-7 99.87%
Reaction Conditions:
with hydrogenchloride in 1,4-dioxane at 20; for 3 h;
Steps:
10.7 Step 7: Synthesis of (2S,4R)-1-((S)-2-amino-3,3-dimethylbutanoyl)-4-hydroxy-N-(4-(4-methylthiazol-5-yl)benzyl)pyrrollidine-2-carboxamide (11)
tert-butyl ((S)-1-((2S,4R)-4-hydroxy-2-((4-(4-methylthiazol-5-yl)benzyl)carbamoyl)pyrrolidine-1- yl)-3,3-dimethyl-1-oxobutan-2-yl) carbamate (19 g, 35.80 mmol) and a mixture of HCl/dioxane (4 M, 100 mL) was stirred at 20° C. for 3 hours. LCMS showed the main peak of the desired mass. The solvent was evaporated to obtain the title compound (16.7 g, 35.76 mmol, 99.87% yield, HCl salt) as a pale yellow solid, It was used directly in the next step without further purification.
References:
KR102160377,2020,B1 Location in patent:Paragraph 0461; 0484-0487
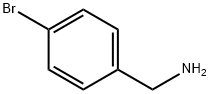
3959-07-7
207 suppliers
$10.00/1g

1448189-80-7
141 suppliers
$20.00/5mg
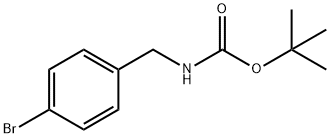
68819-84-1
122 suppliers
$11.00/250mg

1448189-80-7
141 suppliers
$20.00/5mg
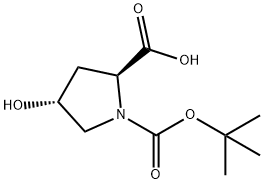
13726-69-7
437 suppliers
$5.00/1g

1448189-80-7
141 suppliers
$20.00/5mg
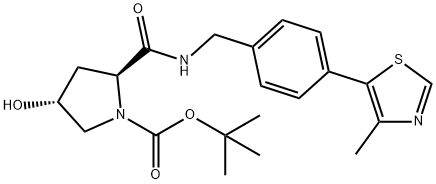
1448191-54-5
14 suppliers
inquiry

1448189-80-7
141 suppliers
$20.00/5mg