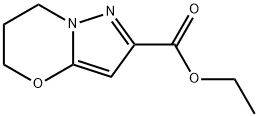
ETHYL 6,7-DIHYDRO-5H-PYRAZOLO[5,1-B][1,3]OXAZINE-2-CARBOXYLATE synthesis
- Product Name:ETHYL 6,7-DIHYDRO-5H-PYRAZOLO[5,1-B][1,3]OXAZINE-2-CARBOXYLATE
- CAS Number:153597-59-2
- Molecular formula:C9H12N2O3
- Molecular Weight:196.2
85230-37-1

109-64-8
![ETHYL 6,7-DIHYDRO-5H-PYRAZOLO[5,1-B][1,3]OXAZINE-2-CARBOXYLATE](/CAS/GIF/153597-59-2.gif)
153597-59-2
Step 1: Preparation of ethyl 6,7-dihydro-5H-pyrazolo[5,1-b][1,3]oxazine-2-carboxylate: To a stirred suspension of ethyl 5-keto-4,5-dihydro-1H-pyrazole-3-carboxylate (10.34 g, 0.66 mol) and potassium carbonate (36.62 g) in acetonitrile (500 mL) was slowly added 1,3-dibromopropane (14.7 g). The reaction mixture was heated to reflux for 16 hours. After completion of the reaction, the mixture was cooled to room temperature, filtered and the solid was washed with acetonitrile. The filtrate and washings were combined and concentrated to dryness under reduced pressure to give an oily residue. The residue was dissolved in ethyl acetate and extracted with water. The organic phase was separated, dried with anhydrous magnesium sulfate, filtered and concentrated under reduced pressure to give the crude product. By further purification, ethyl 6,7-dihydro-5H-pyrazolo[5,1-b][1,3]oxazine-2-carboxylate (8.80 g, 68% yield) was obtained with a melting point of 44-46 °C and a mass spectrum showing the (M + H)+ peak at 197.

85230-37-1
96 suppliers
$58.00/1g

109-64-8
507 suppliers
$10.00/5g
![ETHYL 6,7-DIHYDRO-5H-PYRAZOLO[5,1-B][1,3]OXAZINE-2-CARBOXYLATE](/CAS/GIF/153597-59-2.gif)
153597-59-2
46 suppliers
$55.00/100mg
Yield:153597-59-2 96%
Reaction Conditions:
with potassium carbonate in acetonitrile; for 16 h;Reflux;
Steps:
13.1 Step 1
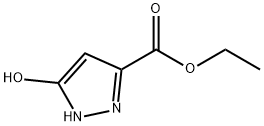
The compound ethyl 5-hydroxy-1H-pyrazole-3-carboxylate (1.0g, 6.40mmol), 1,3-dibromopropane (0.72mL, 7.04mmol) and potassium carbonate (3.54g, 25.65 mmol) was added to acetonitrile (40 mL) and heated to reflux for 16 hours. After the raw material reaction is complete, the reaction solution is cooled to room temperature, filtered to remove insoluble materials, and the filtrate is concentrated to obtain the crude oily product 6,7-dihydro-5H-pyrazolo[5,1-b][1,3] Ethyl oxazine-2-carboxylate (1.81 g, Y: 96%).
References:
CN113429410,2021,A Location in patent:Paragraph 0312-0314

109-64-8
507 suppliers
$10.00/5g

85230-37-1
96 suppliers
$58.00/1g
![ETHYL 6,7-DIHYDRO-5H-PYRAZOLO[5,1-B][1,3]OXAZINE-2-CARBOXYLATE](/CAS/GIF/153597-59-2.gif)
153597-59-2
46 suppliers
$55.00/100mg