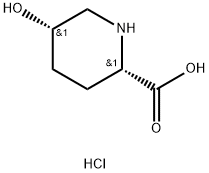
(2S,5S)-5-hydroxypiperidine-2-carboxylic acid hydrochloride synthesis
- Product Name:(2S,5S)-5-hydroxypiperidine-2-carboxylic acid hydrochloride
- CAS Number:154307-84-3
- Molecular formula:C6H12ClNO3
- Molecular Weight:181.62
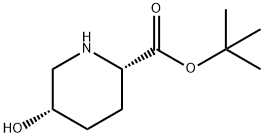
1383814-52-5

154307-84-3
The general procedure for the synthesis of (2S,5S)-5-hydroxypiperidine-2-carboxylic acid hydrochloride from the compound (CAS:1383814-52-5) is as follows: Step 1: Preparation of (2S,5S)-5-hydroxypiperidine-2-carboxylic acid hydrochloride (2S,5S)-5-hydroxypiperidine-2-carboxylic acid tert-butyl ester (126.22 g, 0.63 mol) was gradually added to 5 M hydrochloric acid (630 mL) at room temperature, followed by heating to 65 °C and stirring for 2 hours. Upon completion of the reaction, the reaction solution was cooled to room temperature and the solvent was removed by concentration under reduced pressure. The residue was dissolved in water (500 mL), activated carbon (6.5 g) was added and stirred at room temperature for 30 minutes. The activated carbon was removed by diatomaceous earth filtration and the diatomaceous earth was washed twice with (100 mL) water, the filtrates were combined and concentrated under reduced pressure. The resulting residue (150 g) was cooled in an ice bath, inoculated and stirred. Acetone (650 mL) was added slowly and dropwise over 30 minutes and stirring was continued for 30 minutes. The precipitated crystals were filtered under argon protection and washed with acetone. The crystals were dried under vacuum overnight to give 108.79 g of (2S,5S)-5-hydroxypiperidine-2-carboxylic acid hydrochloride as colorless powdery crystals (96% yield). 1H NMR (400 MHz, D2O) δ 1.66-2.23 (m, 4H), 3.25 (d, J = 3.4 Hz, 1H), 3.38 (d, J = 3.4 Hz, 1H), 4.00 (dd, J = 11.7, 3.7 Hz, 1H), 4.22 (brs, 1H); MS m/z: 146 (M-HCl + H)+.

1383814-52-5
1 suppliers
inquiry

154307-84-3
40 suppliers
$35.00/100mg
Yield:154307-84-3 96%
Reaction Conditions:
with hydrogenchloride in water at 20 - 65; for 2 h;
Steps:
114.1 Step 1 Hydrochloride of (2S,5S)-5-hydroxypiperidine-2-carboxylic acid (7)
Step 1 Hydrochloride of (2S,5S)-5-hydroxypiperidine-2-carboxylic acid (7) [0646] (2S,5S)-tert-Butyl 5-hydroxypiperidine-2-carboxylate (126.22g, 0.63 mol) described in Example 1 was gradually added into 5M hydrochloric acid (630 mL) at room temperature, followed by heating to 65 °C and stirring for 2 hours. Subsequently, the reaction solution was cooled to room temperature and the reaction solvent was concentrated under reduced pressure. The residue was dissolved in water (500 mL), and activated carbon (6.5 g) was added, followed by stirring at room temperature for 30 minutes. The activated carbon was filtered through Celite. Celite was washed twice with water (100 mL) and the filtrate was combined, followed by concentrating under reduced pressure. The residue (150 g) was ice-cooled, followed by inoculation and stirring. To the mixture was added dropwise acetone (650 mL) over 30 minutes, followed by stirring for 30 minutes. The deposited crystals were filtered off under an argon stream, followed by washing with acetone. After deliquoring and drying under vacuum overnight, 108.79 g of the title compound was afforded as a colorless powder crystal (yield 96%). 1H NMR (400 MHz, D2O) δ 1.66-2.23 (m, 4H), 3.25 (d, J = 3.4 Hz, 1H), 3.38 (d, J = 3.4 Hz, 1H), 4.00 (dd, J = 11.7, 3.7 Hz, 1H), 4.22 (brs, 1H); MS m/z: 146 (M-HCl+H)+.
References:
EP2857401,2015,A1 Location in patent:Paragraph 0646
1253856-42-6
7 suppliers
inquiry

154307-84-3
40 suppliers
$35.00/100mg
695183-75-6
16 suppliers
$1561.00/1g

154307-84-3
40 suppliers
$35.00/100mg

138565-95-4
0 suppliers
inquiry

154307-84-3
40 suppliers
$35.00/100mg

133008-09-0
0 suppliers
inquiry

154307-84-3
40 suppliers
$35.00/100mg