
(Z)-Methyl 2-(hydroxyiMino)-2-(pyridin-2-yl)acetate synthesis
- Product Name:(Z)-Methyl 2-(hydroxyiMino)-2-(pyridin-2-yl)acetate
- CAS Number:154410-82-9
- Molecular formula:C8H8N2O3
- Molecular Weight:180.16
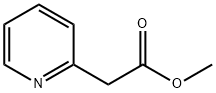
1658-42-0

154410-82-9
The general procedure for the synthesis of methyl 2-(oxime)-2-(pyridin-2-yl)acetate from methyl 2-pyridylacetate was as follows: to a stirring solution of methyl 2-pyridylacetate (8.9 mL, 0.66 mmol) in acetic acid (16 mL) at 0 °C, an aqueous sodium nitrite solution (4.67 g, 0.66 mmol, 14 mL) was slowly added. After addition, the reaction mixture was continued to be stirred at room temperature for 40 min. Subsequently, water (30 mL) was added and the mixture was stirred for another 1 hour. Upon completion of the reaction, the mixture was concentrated to remove most of the acetic acid and alkalized with aqueous sodium carbonate to pH 8-9. The aqueous phase was extracted with ethyl acetate (3×). The organic phases were combined, dried with magnesium sulfate, filtered, and concentrated under vacuum. Finally, the product was dried in a vacuum oven to afford methyl 2-(oxime)-2-(pyridin-2-yl)acetate (11.6 g, 97% yield) as an off-white solid with a mass spectrum m/z 181.6 [M + 1]+.

1658-42-0
185 suppliers
$11.00/1g

154410-82-9
21 suppliers
inquiry
Yield:154410-82-9 97%
Reaction Conditions:
with acetic acid;sodium nitrite in water at 0 - 20; for 1.66667 h;
Steps:
To a solution of pyridin-2-yl-acetic acid methyl ester (8.9 ml, 0.66 mmol) in AcOH (16 mL) at 0 °C with stirring, an aqueous solution of sodium nitrite (4.67 g, 0.66 mmol in 14 mL) was added portion wise. After addition was completed stirring was continued for 40 min at room temperature. Water (30 mL) was added and the mixture was stirred for additional 1 h. The mixture was concentrated to remove most of the AcOH and basidified to pH 8-9 with Na2CO3 aq. extracted with EtOAc (3x). The combined organics were dried over MgSO4, filtered, and concentrated in vacuo. Drying in a vacuum oven affords Hydroxyimino-pyridin-2-yl-acetic acid methyl ester (11.6 g, 97%) as an off-white solid, m/z 181.6 [M + I]+
References:
WO2009/70485,2009,A1 Location in patent:Page/Page column 131

73686-44-9
0 suppliers
inquiry

154410-82-9
21 suppliers
inquiry
61890-12-8
0 suppliers
inquiry

154410-82-9
21 suppliers
inquiry
1620-53-7
31 suppliers
inquiry

154410-82-9
21 suppliers
inquiry

85274-59-5
1 suppliers
inquiry

154410-82-9
21 suppliers
inquiry