
tert-Butyl 6-hydroxy-8-methyl-3,4-dihydroisoquinoline-2(1H)-carboxylate synthesis
- Product Name:tert-Butyl 6-hydroxy-8-methyl-3,4-dihydroisoquinoline-2(1H)-carboxylate
- CAS Number:1579518-38-9
- Molecular formula:C15H21NO3
- Molecular Weight:263.33

24424-99-5
871 suppliers
$13.50/25G

1579518-36-7
0 suppliers
inquiry

1579518-38-9
1 suppliers
inquiry
Yield:1579518-38-9 33.4 g
Reaction Conditions:
in dichloromethane at 15; for 12 h;
Steps:
25 Preparation of intermediate 6-Bromomethyl-8-methyl-3,4-dihydro-1H-isoquinoline-2-carboxylic acid tert-butyl ester (25-14)
To this crude residue is added Boc2O (72 g, 0.33 mol) and triethylamine (63 g, 0.62 mol) and the resulting mixture is stirred for 12 h at 15° C., then diluted with dichloromethane (1500 mL) and water (100 mL).
The organics layer is washed with 0.5 N HCl (100 mL) and brine (100 mL), dried, concentrated, and purified by silica gel column (PE:EtOAc=30:1) to give 6-Hydroxy-8-methyl-3,4-dihydro-1H-isoquinoline-2-carboxylic acid tert-butyl ester, 25-10 (33.4 g) as a white solid.
References:
US2014/73629,2014,A1 Location in patent:Paragraph 0268
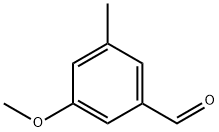
90674-26-3
89 suppliers
$16.00/250mg

1579518-38-9
1 suppliers
inquiry
![Benzene, 1-methoxy-3-methyl-5-[(1E)-2-nitroethenyl]-](/CAS/20210305/GIF/1579518-27-6.gif)
1579518-27-6
0 suppliers
inquiry

1579518-38-9
1 suppliers
inquiry

1000520-77-3
3 suppliers
inquiry

1579518-38-9
1 suppliers
inquiry
![Formamide, N-[2-(3-methoxy-5-methylphenyl)ethyl]-](/CAS/20210305/GIF/1579518-30-1.gif)
1579518-30-1
0 suppliers
inquiry

1579518-38-9
1 suppliers
inquiry