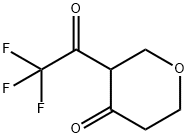
4H-Pyran-4-one, tetrahydro-3-(trifluoroacetyl)- (9CI) synthesis
- Product Name:4H-Pyran-4-one, tetrahydro-3-(trifluoroacetyl)- (9CI)
- CAS Number:158351-87-2
- Molecular formula:C7H7F3O3
- Molecular Weight:196.12
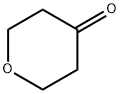
29943-42-8
432 suppliers
$5.00/1g
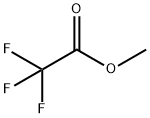
383-63-1
499 suppliers
$10.00/25g

158351-87-2
12 suppliers
inquiry
Yield:158351-87-2 100%
Reaction Conditions:
Stage #1: Tetrahydro-4H-pyran-4-onewith lithium diisopropyl amide in tetrahydrofuran at -70; for 0.5 h;
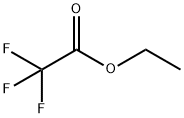
Stage #2: ethyl trifluoroacetate, in tetrahydrofuran at -70 - 20; for 16 h;
Steps:
9
Description 9: 3-(trifluoroacetyl)tetrahydro-4H-pyran-4-one; A solution of tetrahydro-4H-pyran-4-one (5.Og, 50mmol) in tetrahydrofuran (100ml) was cooled to -7O0C with stirring under argon. The solution was then treated with a 2M solution of lithium diisopropylamide in tetrahydrofuran (25ml, 50 mmol) dropwise over 30 minutes. The mix was then stirred at -7O0C for 30 minutes, then treated dropwise with ethyl trifluoroacetate (7.1g, 5.9ml, 50mmol) with stirring under argon. The mix was then allowed to warm slowly up to 2O0C and stirred for 16 hours under argon. The reaction mix was then partitioned between ethyl acetate (50ml) and water (100ml). The organic layer was dried over sodium sulphate and the solvent removed by rotary evaporation to give the title compound as a foamy yellow solid (10.34g, quantitative).LC/MS (ES): Found 195 (ES-), retention time 2.35 mins. C7H7F3O3 requires 196.1 H-NMR (400MHz, MeOD-d4): δ 2.34 (2H, m), 3.22 (1 H, m), 3.87 (2H, m), 4.50 (2H, m).
References:
WO2009/53448,2009,A1 Location in patent:Page/Page column 37