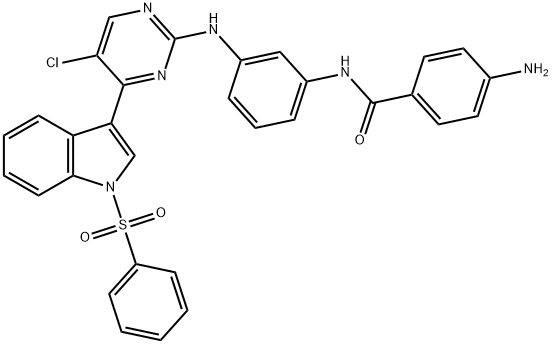
4-amino-N-(3-((5-chloro-4-(1-(phenylsulfonyl)-1H-indol-3-yl)pyrimidin-2-yl)amino)phenyl)benzamide synthesis
- Product Name:4-amino-N-(3-((5-chloro-4-(1-(phenylsulfonyl)-1H-indol-3-yl)pyrimidin-2-yl)amino)phenyl)benzamide
- CAS Number:1604811-59-7
- Molecular formula:C31H23ClN6O3S
- Molecular Weight:595.07
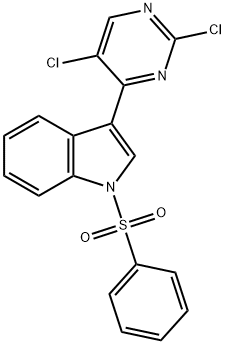
882562-40-5
52 suppliers
$42.00/100mg
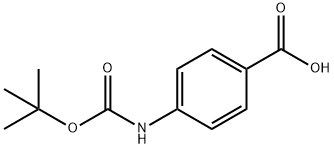
![Carbamic acid, N-[4-[[(3-aminophenyl)amino]carbonyl]phenyl]-, 1,1-dimethylethyl ester](/CAS/20200611/GIF/1604811-57-5.gif)
1604811-57-5
0 suppliers
inquiry

1604811-59-7
14 suppliers
inquiry
Yield:1604811-59-7 35%
Reaction Conditions:
in 1-methyl-pyrrolidin-2-one at 175; for 0.5 h;
Steps:
1.2 4-amino-N-(3-(5-chloro-4-(1-(phenylsulfonyl)- 1H-indol-3-yl)pyrimidin-2- ylamino)phenyl)benzamide
4-amino-N-(3-(5-chloro-4-(1-(phenylsulfonyl)- 1H-indol-3-yl)pyrimidin-2- ylamino)phenyl)benzamide [00373] A solution of 3- (2,5-dichloropyrimidin-4-yl)- 1 -(phenylsulfonyl)- 1 H-indole(350 mg, 0.87 mmol) and tert-butyl 4-(3-aminophenylcarbamoyl)phenylcarbamate (283 mg,0.87 mmol) in NMP (2.3 mL, 0.25M) was heated 30 mm at 175 °C (tW). The cooled mixturewas diluted with EtOAc (20 mL), washed with water (3x5mL), brine (5 mL), dried (MgSO4),filtered, and concentrated under reduced pressure. The residue was purified by Si02 chromatography (DCM/EtOAc 0 to 30% gradient), affording the title compound (180 mg, 0.303 mmol, 35%) as white solid.
References:
WO2014/63068,2014,A1 Location in patent:Paragraph 00373
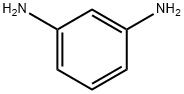
66493-39-8
167 suppliers
$10.00/1g

1604811-59-7
14 suppliers
inquiry

113261-00-0
14 suppliers
$65.00/100mg

1604811-59-7
14 suppliers
inquiry
108-45-2
478 suppliers
$13.00/25g

1604811-59-7
14 suppliers
inquiry
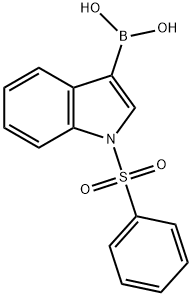
129271-98-3
98 suppliers
$15.00/100mg

1604811-59-7
14 suppliers
inquiry