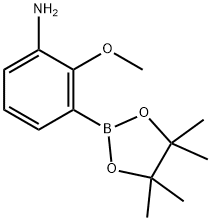
2-Methoxy-3-(4,4,5,5-tetramethyl-[1,3,2]dioxaborolan-2-yl)-phenylamine synthesis
- Product Name:2-Methoxy-3-(4,4,5,5-tetramethyl-[1,3,2]dioxaborolan-2-yl)-phenylamine
- CAS Number:1609393-90-9
- Molecular formula:C13H20BNO3
- Molecular Weight:249.11
116557-46-1
164 suppliers
$9.00/250mg

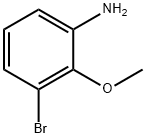
73183-34-3
586 suppliers
$6.00/5g
![2-Methoxy-3-(4,4,5,5-tetramethyl-[1,3,2]dioxaborolan-2-yl)-phenylamine](/CAS/20180703/GIF/1609393-90-9.gif)
1609393-90-9
45 suppliers
$130.00/50mg
Yield: 81%
Reaction Conditions:
with dichloro(1,1'-bis(diphenylphosphanyl)ferrocene)palladium(II)*CH2Cl2;potassium acetate in 1,4-dioxane at 100; for 15 h;Inert atmosphere;
Steps:
3.3 Preparation 3
Step 3[00174j IntlO (2.0 g, 9.9 mmol) was dissolved in dioxane (40 mL) and the vessel purged with nitrogen for 5 minutes. Next bis(pinacolato)diborone (3.77 g, 14.85 mmol), [1,1 ‘-bis(diphenylphosphino)ferrocene] dichloropalladium (II) complex with dichloromethane (404 mg, 0.49 mmol) and potassium acetate (2.91 g, 29.7 mmol) were added. The flask was evacuated and backfilled with nitrogen, and then heated to 100 °Cfor 15 hours. Water was added to quench the reaction and the product was then extracted with EtOAc. The combined organic layers were washed with brine (x3), dried over sodium sulfate, filtered, concentrated and purified using automated chromatography (elutes at 40% ethyl acetate) to provide Intl 1 (2.0 g, 8 1%). ‘H NMR (400MHz, chloroform-d) ö 7.12 (dd,J=7.3, 1.8 Hz, 1H), 6.96-6.89 (m, 1H), 6.88-6.83 (m, 1H),3.82 (s, 3H), 1.37 (s, 12H). LC retention time 0.65 [J]. m/z: 250 (MHj.
References:
BRISTOL-MYERS SQUIBB COMPANY;MOSLIN, Ryan M.;WEINSTEIN, David S.;WROBLESKI, Stephen T.;TOKARSKI, John S.;KUMAR, Amit WO2014/74661, 2014, A1 Location in patent:Paragraph 00174
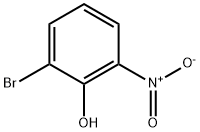
13073-25-1
339 suppliers
$6.00/1g
![2-Methoxy-3-(4,4,5,5-tetramethyl-[1,3,2]dioxaborolan-2-yl)-phenylamine](/CAS/20180703/GIF/1609393-90-9.gif)
1609393-90-9
45 suppliers
$130.00/50mg
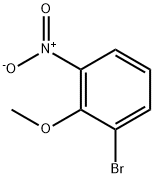
98775-19-0
196 suppliers
$8.00/250mg
![2-Methoxy-3-(4,4,5,5-tetramethyl-[1,3,2]dioxaborolan-2-yl)-phenylamine](/CAS/20180703/GIF/1609393-90-9.gif)
1609393-90-9
45 suppliers
$130.00/50mg
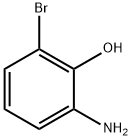
28165-50-6
173 suppliers
$7.00/250mg
![2-Methoxy-3-(4,4,5,5-tetramethyl-[1,3,2]dioxaborolan-2-yl)-phenylamine](/CAS/20180703/GIF/1609393-90-9.gif)
1609393-90-9
45 suppliers
$130.00/50mg