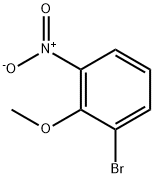
1-Bromo-2-methoxy-3-nitro-benzene synthesis
- Product Name:1-Bromo-2-methoxy-3-nitro-benzene
- CAS Number:98775-19-0
- Molecular formula:C7H6BrNO3
- Molecular Weight:232.03
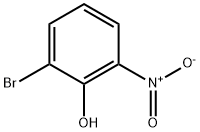
13073-25-1

74-88-4

98775-19-0
General procedure for the synthesis of 2-bromo-6-nitroanisole from 2-bromo-6-nitrophenol (19.6 g, 90.0 mmol) and iodomethane (25.6 g, 180 mmol): first, 2-bromo-6-nitrophenol and potassium carbonate (24.9 g, 180 mmol) were mixed in acetone (400 mL). Subsequently, iodomethane was added to the mixture. The reaction mixture was heated to reflux overnight. After completion of the reaction, it was cooled to room temperature, the mixture was filtered and the filter cake was washed with ethyl acetate (100 mL). The filtrate was concentrated under reduced pressure. The residue was dissolved in ethyl acetate and the resulting solution was washed with saturated brine and dried with anhydrous sodium sulfate. Finally, the dried solution was concentrated to give 1-bromo-2-methoxy-3-nitrobenzene (20.2 g, 97% yield) as a yellow solid. The 1H-NMR spectrum (300 MHz, DMSO-d6) data of the product were as follows: δ (ppm): 3.92 (s, 3H), 7.32 (d, 1H, J = 8.1 Hz), 7.94-8.03 (m, 2H).
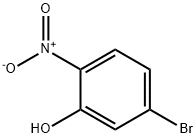
27684-84-0
159 suppliers
$9.00/1g

74-88-4
356 suppliers
$15.00/10g

98775-19-0
196 suppliers
$8.00/250mg
Yield:98775-19-0 76%
Reaction Conditions:
with potassium carbonate in acetone for 24 h;Heating / reflux;
Steps:
3.b b) 2-Bromo-6-nitroanisole
A mixture of the compound from Example 2a) (10.8 g; 0.0495 mol.), methyl iodide (3.4 mL; 0.00545 mol.) and potassium carbonate (8.2 g; 0.0592 mol.) in acetone (250 mL) was stirred and heated under reflux for 24 h. [0392] The mixture was evaporated and the residue triturated with water to afford the title compound (8.7 g; 76%). mp 55-56° C. 1H NMR (300 MHz, CDCl3 δ 7.81-7.74 (m, 2H), 7.13 (t, J=8.1 Hz, 1H), 4.02 (s, 3H); Anal. (C7H6NO3Br) calcd: C, 36.24; H, 2.61; N, 6.04. found: C, 36.30; H, 2.59; N, 5.73.
References:
Erickson-Miller, Connie J;Eppley, Daniel F.;Jenkins, Julian;Luengo, Juan I.;Liu, Nannan;Price, Alan T.;Shaw, Anthony N.;Visonneau, Sophie;Wiggall, Kenneth US2004/19190, 2004, A1 Location in patent:Page 18

13073-25-1
339 suppliers
$6.00/1g

74-88-4
356 suppliers
$15.00/10g

98775-19-0
196 suppliers
$8.00/250mg
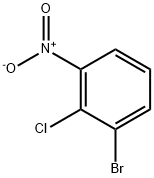
3970-37-4
136 suppliers
$11.00/250mg

124-41-4
730 suppliers
$12.00/25g

98775-19-0
196 suppliers
$8.00/250mg
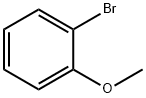
578-57-4
311 suppliers
$6.00/10g
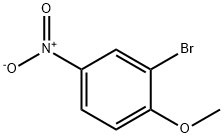
5197-28-4
199 suppliers
$10.00/5g

98775-19-0
196 suppliers
$8.00/250mg

13073-25-1
339 suppliers
$6.00/1g

77-78-1
319 suppliers
$19.69/1ml

98775-19-0
196 suppliers
$8.00/250mg