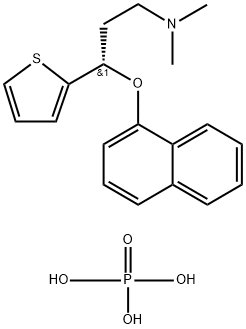
(S)-N,N-DIMETHYL-[3-(2-THIENYL)-3-(1-NAPHTHYLOXY)PROPYL]AMINE--PHOSPHORIC ACID (1:1) synthesis
- Product Name:(S)-N,N-DIMETHYL-[3-(2-THIENYL)-3-(1-NAPHTHYLOXY)PROPYL]AMINE--PHOSPHORIC ACID (1:1)
- CAS Number:161005-84-1
- Molecular formula:C19H24NO5PS
- Molecular Weight:409.44
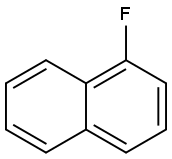
321-38-0
604 suppliers
$6.00/10g
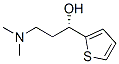
132335-44-5
338 suppliers
$6.00/1g
![(S)-N,N-DIMETHYL-[3-(2-THIENYL)-3-(1-NAPHTHYLOXY)PROPYL]AMINE--PHOSPHORIC ACID (1:1)](/CAS/GIF/161005-84-1.gif)
161005-84-1
17 suppliers
inquiry
Yield:-
Reaction Conditions:
Stage #1: 1-Fluoronaphthalene;(S)-3-dimethylamino-1-(2-thienyl)propan-1-olwith potassium hydroxide in diethylene glycol dimethyl ether at 120; for 3 - 6 h;
Stage #2: with phosphoric acid in water;ethyl acetate; for 1.08333 - 1.25 h;
Steps:
1
EXAMPLE 1; Arylation of (S)-(-)-N,N-dimethyl-3(2-thienyl)-3-hydroxypropanamine To a 25 mL 3-neck round bottom flask equipped with a condenser, N2 inlet, thermocouple, and overhead stirrer charge Compound Ib (0.55 g, 3 mMol), powdered KOH (0.9 g, 14.2 mMol), diglyme (6 mL) and 1-fluoronaphthalene (0.5 mL). Heat the mixture with good agitation to 120° C. for 3 to 6 hrs. Cool to ambient temp. and dilute with water (6 mL) and EtOAc (6 mL). Separate layers, back extract aqueous with EtOAc (6 mL). Separate layers and combine organic layers. Place organic layers in a 25 mL 3-neck round bottom flask with overhead agitation. Slowly add 85% H3PO4 (0.25 mL). Seed after 10 drops have been added. Stir 5-15 minutes, complete acid addition and stir 1 hr. Filter and wash cake with EtOAc (10 mLs). Dry under reduced pressure to yield 1.0 g of the title compound. % ΔR:0.2. Monitor the % R enantiomer by chiral HPLC under the following conditions: Column; Diacel Chiralcel OD-H, 5micron silica, 4.6×250 mm internal diameter, 40° C.; Solvent: 95% hexane, 5% isopropanol+0.2% DEA, 1 mL/min; detection UV 280 nm.
References:
US2007/167636,2007,A1 Location in patent:Page/Page column 4

321-38-0
604 suppliers
$6.00/10g
582-25-2
366 suppliers
$5.00/25g

132335-44-5
338 suppliers
$6.00/1g
![(S)-N,N-DIMETHYL-[3-(2-THIENYL)-3-(1-NAPHTHYLOXY)PROPYL]AMINE--PHOSPHORIC ACID (1:1)](/CAS/GIF/161005-84-1.gif)
161005-84-1
17 suppliers
inquiry