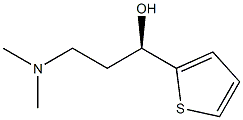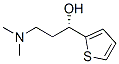
(S)-(-)-N,N-Dimethyl-3-hydroxy-3-(2-thienyl)propanamine synthesis
- Product Name:(S)-(-)-N,N-Dimethyl-3-hydroxy-3-(2-thienyl)propanamine
- CAS Number:132335-44-5
- Molecular formula:C9H15NOS
- Molecular Weight:185.29
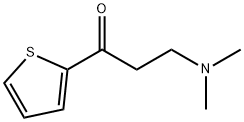
13196-35-5

132335-44-5
General procedure for the synthesis of (S)-N,N-dimethyl-3-hydroxy-3-(2-thienyl)propylamine from N/A (Example 23): under argon protection, (R,R)-Cl2((R)-hexylBINAP)((R)-DAIPEN) (30.5 mg, 0.025 mmol), 2-propanol (100 ml), 1.0 M tert-butyl potassium oxychloride solution of 2-propanol (7.5 ml, 7.5 mmol) and 3-(N,N-dimethylamino)-1-(2-thienyl)-1-propanone (22.9 g, 0.125 mol) were added sequentially to a glass autoclave. After the reaction system was degassed several times and replaced with argon, hydrogen was introduced to a predetermined pressure to start the reaction. After stirring the reaction at 28°C for 6 hours, the reaction system was returned to normal temperature and pressure. The reaction solution was concentrated, heptane was added and the precipitated solid was filtered. The resulting solid was dried under reduced pressure to afford (S)-N,N-dimethyl-3-hydroxy-3-(2-thienyl)propylamine 18.5 g in 80.0% yield. The optical purity of the product was determined by chiral column HPLC, and the result showed that the optical purity was 99% ee. Note: (R)-DAIPEN is (R)-1-isopropyl-2,2-bis(p-methoxyphenyl)ethylenediamine, and xylBINAP is 2,2'-bis(bis-3,5-dimethylphosphinodiphenyl)-1,1'-binaphthyl.
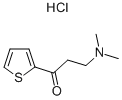
5424-47-5
206 suppliers
$9.00/5g

132335-44-5
338 suppliers
$6.00/1g
Yield:132335-44-5 92.1%
Reaction Conditions:
with glucose dehydrogenase;D-glucose;Rhodosporidium toruloides carbonyl reductase 9 from Escherichia coli;NADPH;sodium hydroxide in aq. phosphate buffer; pH=7 at 30; for 4 h;Kinetics;Enzymatic reaction;enantioselective reaction;Temperature;
Steps:
In a 2-L bioreactor, 2a (1000mM), glucose (6000mM), NADP+ (0.75mM), and sonicated cells of co-expressing E. coli/RtSCR9-GDH (10gDCWL-1) were mixed in a total volume of 1L phosphate buffer (100mM, pH 7.0). The resulting mixture was stirred at 30°C with 200rpm shaking for 240min. The pH was maintained at 7.0 with 2M NaOH during the reaction. After reaching completion, the pH was adjusted to 11-12 followed by extraction using ethyl acetate. The combined organic phases were dried over anhydrous sodium sulfate and concentrated to give (S)- 3a. Yield: 92.1%. 1H NMR (500MHz, DMSO-d6): δ=7.36 (dd, J=4.9, 1.2Hz, 1H), 6.93 (m, 2H), 5.79 (s, 1H), 4.86 (m, 1H), 2.30 (qt, J=12.0, 7.1Hz, 2H), 2.12 (s, 6H), 1.79 (m, 2H). 13C NMR (126MHz, DMSO-d6): δ=150.72, 126.40, 123.79, 122.51, 67.39, 55.96, 45.15, 36.92. [α]25D=-4.9° (c=10mg/mL, ethyl acetate).
References:
Chen, Xiang;Liu, Zhi-Qiang;Lin, Chao-Ping;Zheng, Yu-Guo [Bioorganic Chemistry,2016,vol. 65,p. 82 - 89]

13196-35-5
27 suppliers
inquiry

132335-44-5
338 suppliers
$6.00/1g

124-40-3
545 suppliers
$18.00/100ml
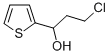
164071-56-1
11 suppliers
$412.00/1g

132335-44-5
338 suppliers
$6.00/1g
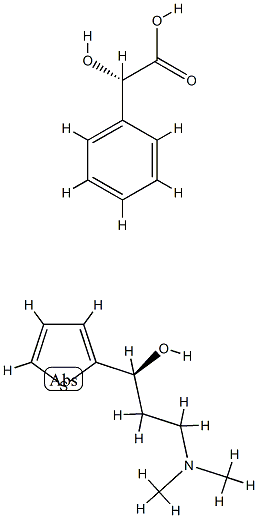
287737-72-8
1 suppliers
inquiry

132335-44-5
338 suppliers
$6.00/1g