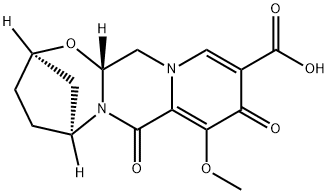
(2R,5S,13aR)-8-methoxy-7,9-dioxo-2,3,4,5,7,9,13,13a-octahydro-2,5-methanopyrido[1',2':4,5]pyrazino[2,1-b][1,3]oxazepine-10-carboxylic acid synthesis
- Product Name:(2R,5S,13aR)-8-methoxy-7,9-dioxo-2,3,4,5,7,9,13,13a-octahydro-2,5-methanopyrido[1',2':4,5]pyrazino[2,1-b][1,3]oxazepine-10-carboxylic acid
- CAS Number:1616342-45-0
- Molecular formula:C15H16N2O6
- Molecular Weight:320.3
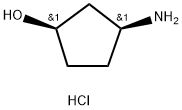
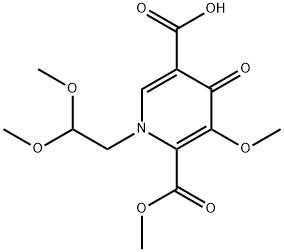
1279032-31-3
170 suppliers
$39.00/100mg
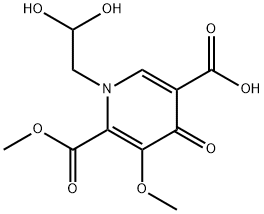
1616340-75-0
2 suppliers
inquiry
![(2R,5S,13aR)-8-methoxy-7,9-dioxo-2,3,4,5,7,9,13,13a-octahydro-2,5-methanopyrido[1',2':4,5]pyrazino[2,1-b][1,3]oxazepine-10-carboxylic acid](/CAS/20180703/GIF/1616342-45-0.gif)
1616342-45-0
28 suppliers
inquiry
Yield:1616342-45-0 80.67 g
Reaction Conditions:
with anhydrous potassium acetate in acetonitrile at 30 - 35; for 10 h;
Steps:
2 Preparation of crude compound of Formula I
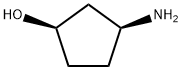
MDHC (100 gm), acetonitrile (2 lit) were added in to a round bottom flask at 25-30°C and stirred for 10 min at same temperature. To the reaction mass was added acetic acid (100 mL) and methane sulfonic acid (3.7 gm) one by one at 25-30°C and heated to 75-80°C and stirred for 18 hrs at same temperature. Then the reaction mass was cool to 30-35°C and was added (1R, 2S)-3-amino-cyclopentanol hydrochloride (43.6 gm) and potassium acetate (110 gm) at same temperature and stirred for 10 hrs. After completion of the reaction, reaction mass was concentrated under vacuum at below 60°C and allowed to cool to 30-35°C to obtain a solid. The solid was dissolved in methylene chloride (500 mL) and was treated with water (500 mL) and sodium chloride solution (500 mL). Organic layer was separated and concentrated under vacuum at below 50°C to obtain a solid. The obtained solid was recrystallized from isopropyl alcohol (600 mL) and dryed under vacuum at 55°C for 8 hrs to obtain title compound. Wt: 80.67 gm. HPLC Purity: 95.1%; Formula IA: 1.5% by HPLC; Formula IB: 2.9% by HPLC.
References:
WO2022/157561,2022,A1 Location in patent:Page/Page column 3; 31
1110772-05-8
96 suppliers
$28.00/10mg

1616340-75-0
2 suppliers
inquiry
![(2R,5S,13aR)-8-methoxy-7,9-dioxo-2,3,4,5,7,9,13,13a-octahydro-2,5-methanopyrido[1',2':4,5]pyrazino[2,1-b][1,3]oxazepine-10-carboxylic acid](/CAS/20180703/GIF/1616342-45-0.gif)
1616342-45-0
28 suppliers
inquiry
1335210-23-5
214 suppliers
$5.00/250mg

1279032-31-3
170 suppliers
$39.00/100mg
![(2R,5S,13aR)-8-methoxy-7,9-dioxo-2,3,4,5,7,9,13,13a-octahydro-2,5-methanopyrido[1',2':4,5]pyrazino[2,1-b][1,3]oxazepine-10-carboxylic acid](/CAS/20180703/GIF/1616342-45-0.gif)
1616342-45-0
28 suppliers
inquiry

1335210-23-5
214 suppliers
$5.00/250mg

1110772-05-8
96 suppliers
$28.00/10mg
![(2R,5S,13aR)-8-methoxy-7,9-dioxo-2,3,4,5,7,9,13,13a-octahydro-2,5-methanopyrido[1',2':4,5]pyrazino[2,1-b][1,3]oxazepine-10-carboxylic acid](/CAS/20180703/GIF/1616342-45-0.gif)
1616342-45-0
28 suppliers
inquiry

1335210-23-5
214 suppliers
$5.00/250mg
![(2R,5S,13aR)-8-methoxy-7,9-dioxo-2,3,4,5,7,9,13,13a-octahydro-2,5-methanopyrido[1',2':4,5]pyrazino[2,1-b][1,3]oxazepine-10-carboxylic acid](/CAS/20180703/GIF/1616342-45-0.gif)
1616342-45-0
28 suppliers
inquiry