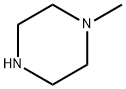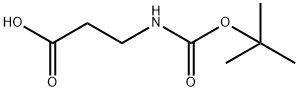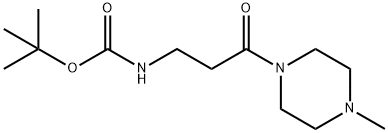
Carbamic acid, N-[3-(4-methyl-1-piperazinyl)-3-oxopropyl]-, 1,1-dimethylethyl ester synthesis
- Product Name:Carbamic acid, N-[3-(4-methyl-1-piperazinyl)-3-oxopropyl]-, 1,1-dimethylethyl ester
- CAS Number:1616684-41-3
- Molecular formula:C13H25N3O3
- Molecular Weight:271.36
Yield:1616684-41-3 70%
Reaction Conditions:
with benzotriazol-1-ol;dicyclohexyl-carbodiimide in tetrahydrofuran at 0 - 20; for 4 h;
Steps:
6 General procedure for the synthesis of 12a-12j
General procedure: Amino acids (10a-10j) were converted to the corresponding Boc derivatives (11a-11j) in quantitative yields by using di-tertbutylpyrocarbonate.18 To a solution of (11a-11j; 2 mmol) in10 mL THF, 1-hydroxybenzotriazole (HOBt; 2.2 mmol) was added. The reaction mixture was stirred for 2 min at 0 °C. The N-methylpiperazine (3 mmol) was added to above reaction mixture, followed by addition of dicyclohexylcarbodimide (DCC; 2.2 mmol,1.0 mL THF) at 0 °C.10 The reaction mixture was allowed to reach at room temperature and was stirred for next 4 h. The dicyclohexylurea (DCU) was filtered and the filtrate was evaporated under reduced pressure. The oily residue was dissolved in chloroform, organic layer was washed with 5% aqueous sodium bicarbonate followed by washing with water and finally with brine. The organic layer was dried over anhydrous Na2SO4 and evaporated under reduced pressure. The crude product was purified over silica gel column to afford compounds (12a-12j) in good yields.
References:
Sinha, Manish;Dola, Vasanth R.;Agarwal, Pooja;Srivastava, Kumkum;Haq, Wahajul;Puri, Sunil K.;Katti, Seturam B. [Bioorganic and Medicinal Chemistry,2014,vol. 22,# 14,p. 3573 - 3586]

24424-99-5
863 suppliers
$13.50/25G
![Carbamic acid, N-[3-(4-methyl-1-piperazinyl)-3-oxopropyl]-, 1,1-dimethylethyl ester](/CAS/20210111/GIF/1616684-41-3.gif)
1616684-41-3
4 suppliers
inquiry