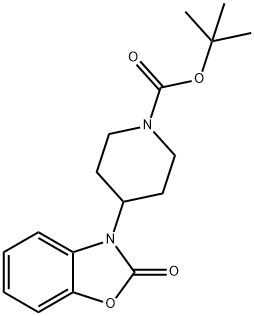
tert-Butyl 4-(2-oxobenzo[d]oxazol-3(2H)-yl)piperidine-1-carboxylate synthesis
- Product Name:tert-Butyl 4-(2-oxobenzo[d]oxazol-3(2H)-yl)piperidine-1-carboxylate
- CAS Number:162045-53-6
- Molecular formula:C17H22N2O4
- Molecular Weight:318.37

79099-07-3
0 suppliers
$5.00/5g
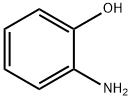
95-55-6
554 suppliers
$5.00/5G
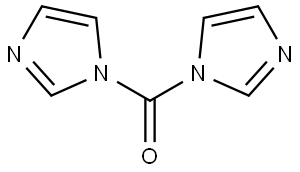
530-62-1
976 suppliers
$6.00/25g
![tert-Butyl 4-(2-oxobenzo[d]oxazol-3(2H)-yl)piperidine-1-carboxylate](/CAS/GIF/162045-53-6.gif)
162045-53-6
24 suppliers
$291.00/1g
Yield:162045-53-6 38%
Reaction Conditions:
Stage #1: N-tert-butyloxycarbonylpiperidin-4-one;2-amino-phenolwith sodium tris(acetoxy)borohydride in dichloromethane at 20; for 14 h;Inert atmosphere;
Stage #2: 1,1'-carbonyldiimidazole in dichloromethane; for 16 h;
Steps:
1.1 Step 1.1. Synthesis of tert-butyl 4-(2-oxobenzo[d]oxazole-3(2H)-yl)piperidine-l-carboxylate
To a stirred solution of tert-butyl 4-oxy-l-piperidine carboxylate (20g, 100 mmol) and 2-aminophenol (11g, 100 mmol) in CH2Cl2 (300 mL), NaBH(OAc)3 (32.4 g, 151 mmol) was added under nitrogen at room temperature.
The mixture was stirred at room temperature for 14 h, diluted by adding CH2Cl2 (1000 mL) and saturated NaHCO3 (1500 mL), and separated into layers.
The aqueous layer was extracted with CH2Cl2 (2* 500ml), the combined organic layer was washed with brine, dried over Na2SO4, and the solvent was removed under reduced pressure.
The crude material was diluted with CH2Cl2 (300 mL), added with carbonyldiimidazole (18 g, 110 mmol), stirred for 16 h, diluted with CH2Cl2 (1 L) and IN HCl (1 L), and separated into layers.
The aqueous layer was extracted with CH2Cl2 (2* 500ml), the combined organic layer was washed with brine, dried over Na2SO4, and the solvent was removed under reduced pressure.
Purification was performed by silica gel column chromatography (PE: EA = 15:1) to obtain a yellow solid (18.4 g, yield 38%). MS (ESI) m/z 319.0 [M + H]+.
References:
EP3960736,2022,A1 Location in patent:Paragraph 0357-0359
32315-10-9
442 suppliers
$10.00/1g
![1-BOC-4-[(2-HYDROXYPHENYL)AMINO]-PIPERIDINE](/CAS/GIF/162045-48-9.gif)
162045-48-9
22 suppliers
$179.30/1g
![tert-Butyl 4-(2-oxobenzo[d]oxazol-3(2H)-yl)piperidine-1-carboxylate](/CAS/GIF/162045-53-6.gif)
162045-53-6
24 suppliers
$291.00/1g

95-55-6
554 suppliers
$5.00/5G
![tert-Butyl 4-(2-oxobenzo[d]oxazol-3(2H)-yl)piperidine-1-carboxylate](/CAS/GIF/162045-53-6.gif)
162045-53-6
24 suppliers
$291.00/1g

79099-07-3
0 suppliers
$5.00/5g
![tert-Butyl 4-(2-oxobenzo[d]oxazol-3(2H)-yl)piperidine-1-carboxylate](/CAS/GIF/162045-53-6.gif)
162045-53-6
24 suppliers
$291.00/1g