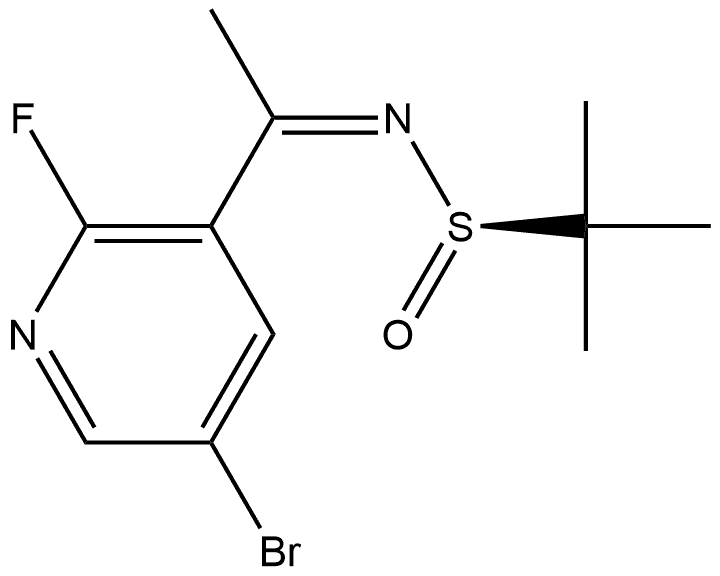
2-Propanesulfinamide, N-[1-(5-bromo-2-fluoro-3-pyridinyl)ethylidene]-2-methyl-, [N(Z),S(R)]- synthesis
- Product Name:2-Propanesulfinamide, N-[1-(5-bromo-2-fluoro-3-pyridinyl)ethylidene]-2-methyl-, [N(Z),S(R)]-
- CAS Number:1620980-59-7
- Molecular formula:C11H14BrFN2OS
- Molecular Weight:321.21
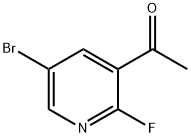
1111637-74-1
80 suppliers
$15.00/250mg
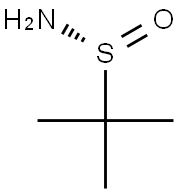
196929-78-9
390 suppliers
$4.00/1g
![2-Propanesulfinamide, N-[1-(5-bromo-2-fluoro-3-pyridinyl)ethylidene]-2-methyl-, [N(Z),S(R)]-](/CAS/20211123/GIF/1620980-59-7.gif)
1620980-59-7
1 suppliers
inquiry
Yield:1620980-59-7 99%
Reaction Conditions:
with titanium(IV) tetraethanolate in tetrahydrofuran; for 2 h;Reflux;
Steps:
23.3 (R,Z)-N-(1-(5-bromo-2-fluoropyridin-3-yl)ethylidene)-2-methylpropane-2-sulfinamide
A mixture of 1-(5-bromo-2-fluoropyridin-3-yl)ethanone (11 g, 51 mmol), (R)-2-methylpropane-2-sulfinamide (12.2 g, 101 mmol) and titanium (IV) ethoxide (26.1 mL, 126 mmol) in THF (100 mL) was heated to reflux for 2 h. The mixture was cooled to room temperature, brine was added and the mixture was stirred for 10 min. The suspension was filtered through silica gel. The organic phase was separated and the aqueous phase was extracted with EtOAc. The combined organic extracts were washed with brine, dried over Na2SO4, filtered, and concentrated. Purification by flash column chromatography on silica gel (eluted with 0-20% EtOAc/hexanes) gave (R,Z)-N-(1-(5-bromo-2-fluoropyridin-3-yl)ethylidene)-2-methylpropane-2-sulfinamide (16 g, 50 mmol, 99% yield) as a bright yellow oil. LC/MS (ESI) m/z=321 (M+H).
References:
US2014/213581,2014,A1 Location in patent:Paragraph 0685
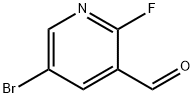
875781-15-0
182 suppliers
$6.00/1g
![2-Propanesulfinamide, N-[1-(5-bromo-2-fluoro-3-pyridinyl)ethylidene]-2-methyl-, [N(Z),S(R)]-](/CAS/20211123/GIF/1620980-59-7.gif)
1620980-59-7
1 suppliers
inquiry

1111637-73-0
26 suppliers
inquiry
![2-Propanesulfinamide, N-[1-(5-bromo-2-fluoro-3-pyridinyl)ethylidene]-2-methyl-, [N(Z),S(R)]-](/CAS/20211123/GIF/1620980-59-7.gif)
1620980-59-7
1 suppliers
inquiry