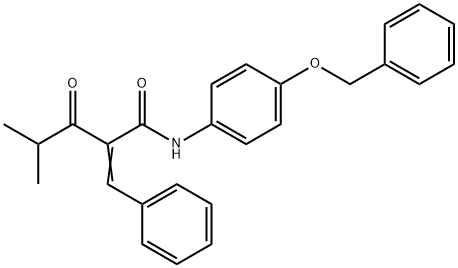
N-4-Benzyloxyphenyl α-Benzilidene Isobutyrylacetamide synthesis
- Product Name:N-4-Benzyloxyphenyl α-Benzilidene Isobutyrylacetamide
- CAS Number:163217-66-1
- Molecular formula:C26H25NO3
- Molecular Weight:399.48

100-52-7
976 suppliers
$20.37/250g

265989-30-8
23 suppliers
inquiry

163217-66-1
14 suppliers
inquiry
Yield:163217-66-1 46.2%
Reaction Conditions:
with acetic acid;3-amino propanoic acid in hexanes; for 28 h;Heating / reflux;
Steps:
H.9.B
The reaction was run in a 1 L three necked round bottomed flask equipped with an electric motor-driven mechanical stirrer and an oil bath. The flask was fitted with a Dean- Stark trap and a condenser, and the entire apparatus was maintained under nitrogen. The flask EPO
References:
WO2006/110918,2006,A1 Location in patent:Page/Page column 57; 58
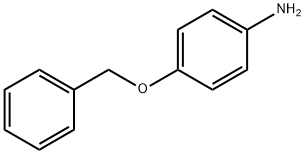
6373-46-2
161 suppliers
$6.00/1g

163217-66-1
14 suppliers
inquiry
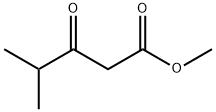
42558-54-3
390 suppliers
$10.00/5g

163217-66-1
14 suppliers
inquiry
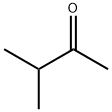
563-80-4
316 suppliers
$18.00/25mL

163217-66-1
14 suppliers
inquiry