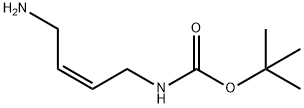
Carbamic acid, (4-amino-2-butenyl)-, 1,1-dimethylethyl ester, (Z)- (9CI) synthesis
- Product Name:Carbamic acid, (4-amino-2-butenyl)-, 1,1-dimethylethyl ester, (Z)- (9CI)
- CAS Number:167845-56-9
- Molecular formula:C9H18N2O2
- Molecular Weight:186.25

24424-99-5
861 suppliers
$13.50/25G

40794-72-7
0 suppliers
inquiry

167845-56-9
9 suppliers
inquiry
![C,C′-Bis(1,1-dimethylethyl) N,N′-(2Z)-2-butene-1,4-diylbis[carbamate]](/CAS/20240320/GIF/215167-64-9.gif)
215167-64-9
0 suppliers
inquiry
Yield:167845-56-9 29.7% ,215167-64-9 38.16%
Reaction Conditions:
in ethanol at 20;
Steps:
3 Synthesis of compound 16 (Z)-(4-aminobut-2-en-1-yl) tert-butyl carbamate
Benzylamine (2L) was added to the 5L reaction flask,Add compound 14(Z)-l,4-diphthalimide-2-butene (262.02g, 0.76mol) into the reaction kettle,Warm up and stir to dissolve,The reaction was carried out at 100°C for 48 hours.Decrease the internal temperature of the reaction to 10°C,Add water (2L) under vigorous stirring,After stirring for 2 hours, filter under reduced pressure,The filter cake was rinsed with 500ml water.The filtrate was extracted with dichloromethane (2L x 1,1Lx3),An aqueous solution containing compound 15(Z)-1,4-diamine-2-butene was obtained.Add the above-mentioned water phase and ethanol (2L) to a 5L reactor.At 20°C,(BOC)2O (116.15g, 0.53mol) was dissolved in ethanol (500ml) and slowly dropped into the reactor,The dripping time is not less than 180 minutes.After the dropwise addition was completed, the reaction was carried out at 20°C for 15 hours.Concentrate under reduced pressure to remove ethanol in the reaction system,The reaction liquid was filtered under reduced pressure,The filter cake was rinsed with 2L water.The filtrate was extracted with DCM (1Lx 1,500mlx3), and the organic phase was concentrated to dryness to give a yellow oil compound 16(Z)-(4-aminobut-2-en-1-yl) tert-butyl carbamate (42.11g, 0.22) mol),Yield 29.7%).Add (BOC)2O (120.08g, 0.55mol) and ethanol (2L) to the water phase,React at room temperature for 15 hours,After removing the ethanol in the reaction system under reduced pressure,Decompression filtration,The filter cake was rinsed with 2L water.The above filter cakes were combined and dried to obtain a white solid compound 17(Z)-di-tert-butylbut-2-ene-1,4-diyl dicarbamate (81.55g, 0.29mol),The yield was 38.16%.
References:
CN113444023,2021,A Location in patent:Paragraph 0129-0130; 0136-0142