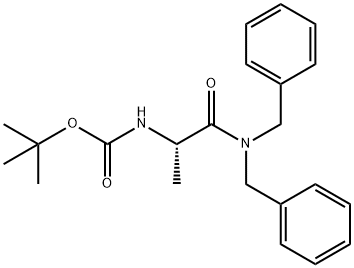
Carbamic acid, [2-[bis(phenylmethyl)amino]-1-methyl-2-oxoethyl]-, 1,1-dimethylethyl ester, (S)- (9CI) synthesis
- Product Name:Carbamic acid, [2-[bis(phenylmethyl)amino]-1-methyl-2-oxoethyl]-, 1,1-dimethylethyl ester, (S)- (9CI)
- CAS Number:170033-50-8
- Molecular formula:C22H28N2O3
- Molecular Weight:368.47
Yield:170033-50-8 63%
Reaction Conditions:
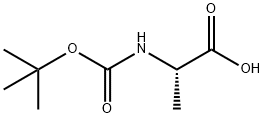
Stage #1: L-N-Boc-Alawith isobutyl chlorocarbonate;triethylamine in tetrahydrofuran at -30 - 20; for 5.5 h;
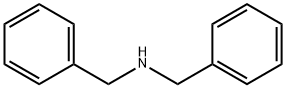
Stage #2: dibenzylaminewith triethylamine in tetrahydrofuran at 0 - 20; for 48 h;
Steps:
24.1 Step 1. Synthesis of tert-butyl (5)-(l-(dibenzylamino)-l-oxopropan-2- ylcarbamate.
To a -30 °C solution of (tert-butoxycarbonyl)-L-alanine (5.00 g, 26.0 mmol) in THF (100 mL) was added TEA (3.20 g, 31.0 mmol) and reaction mixture was added ClC02Bu-z (3.97 g, 29.0 mmol) dropwise. The reaction mixture was stirred at the same temperature for 30 min and then at room temperature for 5 h. The reaction mixture was cooled to 0 °C followed by dropwise addition of dibenzylamine (5.73 g, 29.0 mmol) and TEA (5.73 g, 33.0 mmol) solution in THF (25 mL). The reaction mixture was stirred at room temperature for 48 h. The reaction mixture was quenched with saturated NaHC03 (100 mL) and extracted with EtOAc (3 x 50 mL). The organic layer was separated, washed with brine (20 mL), dried over anhydrous Na2S04, and concentrated in vacuo. The crude obtained was purified by column chromatography (silica 100- 200 mesh, 1 to 10% EtOAc in hexanes) and then triturated with hexanes (5 mL) to afford tert- butyl (S)-(l-(dibenzylamino)-l-oxopropan-2-yl)carbamate (6.17 g, 63%) as a white solid. MS (ESI) m/e [M+H]+/Rt/%: 369.00/3.66/97.2%.
References:
WO2017/20010,2017,A1 Location in patent:Paragraph 0181
15761-38-3
530 suppliers
$5.00/10g
![Carbamic acid, [2-[bis(phenylmethyl)amino]-1-methyl-2-oxoethyl]-, 1,1-dimethylethyl ester, (S)- (9CI)](/CAS/20210111/GIF/170033-50-8.gif)
170033-50-8
0 suppliers
inquiry
![L-Alanine, N-[(1,1-dimethylethoxy)carbonyl]-, anhydride with 2-methylpropyl hydrogen carbonate](/CAS/20211123/GIF/109208-68-6.gif)