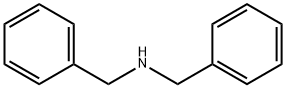
Dibenzylamine synthesis
- Product Name:Dibenzylamine
- CAS Number:103-49-1
- Molecular formula:C14H15N
- Molecular Weight:197.28
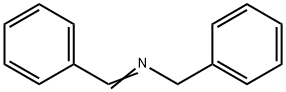
780-25-6
52 suppliers
$61.10/5g

103-49-1
473 suppliers
$5.00/25g
Yield:103-49-1 100%
Reaction Conditions:
with hydrogen;Ru((R,R)-cyP2N2)HCl in benzene-d6 at 20; under 2280.15 Torr; for 4 h;Product distribution / selectivity;Alkaline conditions;Cooling with liquid nitrogen;
Steps:
2.b.7
b) Under an atmosphere of hydrogen gas (1-3 atm) at room temperature, catalytic amounts of the [Ru(P2N2)Y2] or [Ru(P2(NH)2)Y2] complex, together with 3-10 equivalents of KOiPr or KOtBu effectively and readily catalyzed the hydrogenation of the neat ketones or imines to the corresponding alcohol or amine respectively. A general procedure for a catalytic run is as follows: [0074] 1 to 8 g of the substrate, or its solution in 1-2 ml of C6D6, were added under a flow of hydrogen gas to a Schlenk flask containing the desired amount of catalyst and of base (KOiPr or KOtBu). The flask was then cooled to liquid nitrogen temperature, filled with H2 gas, closed and allowed to gradually warm to room temperature to reach an initial H2 pressure of about 3 atm. The mixture was vigorously stirred for 12 to 30 hours. Then, the catalyst was oxidized and precipitated from the alcohols or amines by the addition of hexanes in the air and then removed by filtration through a 5 mm thick pad of silica gel. The hexanes were evaporated to yield the pure alcohol. A sample was dissolved in C6D6 to determine the yield by 1H NMR. Typical conditions and results are listed in Table 5.
References:
US2004/15017,2004,A1 Location in patent:Page 5
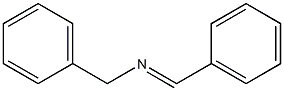
27845-50-7
14 suppliers
inquiry

103-49-1
473 suppliers
$5.00/25g
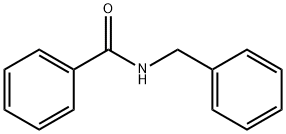
1485-70-7
113 suppliers
$8.00/1g

103-49-1
473 suppliers
$5.00/25g

100-44-7
658 suppliers
$13.50/250G

103-49-1
473 suppliers
$5.00/25g

