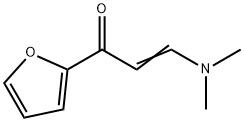
(E)-3-(dimethylamino)-1-(furan-2-yl)prop-2-en-1-one synthesis
- Product Name:(E)-3-(dimethylamino)-1-(furan-2-yl)prop-2-en-1-one
- CAS Number:17168-45-5
- Molecular formula:C9H11NO2
- Molecular Weight:165.19
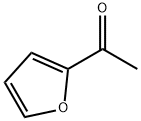
1192-62-7
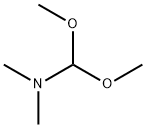
4637-24-5

17168-45-5
General procedure: 2-acetylfuran (25.0 g, 0.227 mol) was mixed with N,N-dimethylformamide dimethyl acetal (40 mL) and the reaction was stirred at 100 °C for 9 hours. Upon completion of the reaction, the mixture was cooled to room temperature and subsequently concentrated to remove the volatile components. Ether and hexane were added to the concentrated residue and the precipitated solid product was collected by filtration. The solid was washed with hexane to give the final 3-(dimethylamino)-1-(furan-2-yl)prop-2-en-1-one (36.5 g, 97% yield) as a brown solid. 3.4 Hz), 7.68 (1H, double peaks, J = 12.6 Hz), 7.79 (1H, double double heavy peaks, J = 0.8, 2.0 Hz).
Yield:17168-45-5 97%
Reaction Conditions:
at 100; for 9 h;
Steps:
14 Reference Example 14: 3-(Dimethylamino)-1-(2-furyl)-2-propen-1-one
A mixture of 2-acetylfuran (25.0 g, 0.227 mmol) and N,N-dimethylformamide dimethylacetal (40 ml) was stirred at 100°C for 9 hours. After cooling as it was, the reaction mixture was concentrated. To the residue were added diethyl ether and hexane. The resulting solid was collected by filtration and washed with hexane, to give the title compound (36.5 g, 97%) as a brown solid.1H NMR (400 MHz, DMSO-d6) δ ppm; 2.88 (3H, br s), 3.14 (3H, br s), 5.65 (1H, d, J= 12.6 Hz), 6.60 (1H, dd, J=2.0, 3.4 Hz), 7.10 (1H, dd, J = 0.8, 3.4 Hz), 7.68 (1H, d, J = 12.6 Hz), 7.79 (1H, dd, J = 0.8, 2.0 Hz).
References:
EP1439175,2004,A1 Location in patent:Page 45-46