
(S)-4-Ethyl-4-hydroxy-6-iodo-8-Methoxy-1,4-dihydro-pyrano[3,4-c]pyridin-3-one synthesis
- Product Name:(S)-4-Ethyl-4-hydroxy-6-iodo-8-Methoxy-1,4-dihydro-pyrano[3,4-c]pyridin-3-one
- CAS Number:174092-79-6
- Molecular formula:C11H12INO4
- Molecular Weight:349.12
![(S)-4-Ethyl-4-hydroxy-8-Methoxy-6-triMethylsilanyl-1,4-dihydro-pyrano[3,4-c]pyridin-3-one](/CAS/GIF/174092-78-5.gif)
174092-78-5
0 suppliers
inquiry
![(S)-4-Ethyl-4-hydroxy-6-iodo-8-Methoxy-1,4-dihydro-pyrano[3,4-c]pyridin-3-one](/CAS/GIF/174092-79-6.gif)
174092-79-6
0 suppliers
inquiry
Yield:174092-79-6 97%
Reaction Conditions:
with iodine;silver trifluoroacetate in dichloromethane at 20; for 17 h;
Steps:
23 Example 23; Synthesis of Compound (q)
To a solution of Compound (p) (50.2 g) in the solvent (about 400 mL; given in Table 15), the reagents in the table were added and the resulting mixture was stirred at the given temperature for the hours. To the reaction mixture 20% sodium carbonate (1.7 L), 10% sodium sulfite (1.0 L) and chloroform (550mL) were added with stirring. The organic layer was separated and the aqueous layer was extracted with chloroform (550 mL × 2). The extracts were combined, dried over anhydrous sodium sulfate, filtered and then evaporated under reduced pressure to dryness. The content of Compound (q) in the residue was quantified by HPLC. The results are summarized in Table 15. This conversion was satisfactorily performed by using NCS - NaI at 65°C in acetic acid (Exp. 39). The period required for the completion was apparently shortened and the yields of Compound (q) under the conditions were higher than that of the report [Comparative Experiment 1 (Com. 1)] by 50% or more. [[Table 15]] SolventReagentEq.TemperatureTime (hr)Yield (%)Com. 1*)ICI4RT to 40 °C4845Exp. 35AcOHNIS1265 °C4563Exp. 36CH2Cl2 I2- CF3CO2Ag2Ambient1797Exp. 37AcOHNCS - NaI665°C1695Exp. 38AcOHNCS - NaI665 °C1693Exp. 39AcOHNCS - NaI665 °C1594*) a mixture of dichloromethane and chloroform (3:2), AcOH: acetic acid, ICl: iodine monochloride, NIS: N-iodosuccinimide, NCS: N-chlorosuccinimide, Eq.: molar ratio of the reagent(s) employed, Yield: isolated yields.
References:
EP1378505,2004,A1 Location in patent:Page 34-35
![4-Pyridineacetonitrile, 3-[[[(1,1-dimethylethyl)dimethylsilyl]oxy]methyl]-α-ethyl-2-methoxy-6-(trimethylsilyl)-α-[(trimethylsilyl)oxy]-, (αS)-](/CAS/20210305/GIF/375346-03-5.gif)
375346-03-5
0 suppliers
inquiry
![(S)-4-Ethyl-4-hydroxy-6-iodo-8-Methoxy-1,4-dihydro-pyrano[3,4-c]pyridin-3-one](/CAS/GIF/174092-79-6.gif)
174092-79-6
0 suppliers
inquiry
![3H-Pyrano[3,4-c]pyridin-3-one, 4-ethyl-1,4-dihydro-4-hydroxy-8-methoxy-6-(trimethylsilyl)-](/CAS/20210305/GIF/869097-09-6.gif)
869097-09-6
0 suppliers
inquiry
![(S)-4-Ethyl-4-hydroxy-6-iodo-8-Methoxy-1,4-dihydro-pyrano[3,4-c]pyridin-3-one](/CAS/GIF/174092-79-6.gif)
174092-79-6
0 suppliers
inquiry
![4-Pyridineacetonitrile, 3-[[[(1,1-dimethylethyl)dimethylsilyl]oxy]methyl]-α-ethyl-6-iodo-2-methoxy-α-[(trimethylsilyl)oxy]-, (αS)-](/CAS/20210305/GIF/375346-04-6.gif)
375346-04-6
0 suppliers
inquiry
![(S)-4-Ethyl-4-hydroxy-6-iodo-8-Methoxy-1,4-dihydro-pyrano[3,4-c]pyridin-3-one](/CAS/GIF/174092-79-6.gif)
174092-79-6
0 suppliers
inquiry
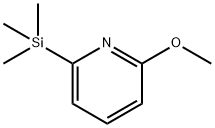
170453-55-1
4 suppliers
inquiry
![(S)-4-Ethyl-4-hydroxy-6-iodo-8-Methoxy-1,4-dihydro-pyrano[3,4-c]pyridin-3-one](/CAS/GIF/174092-79-6.gif)
174092-79-6
0 suppliers
inquiry