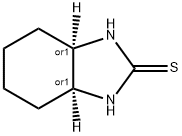
2H-Benzimidazole-2-thione, octahydro-, (3aR,7aS)-rel- synthesis
- Product Name:2H-Benzimidazole-2-thione, octahydro-, (3aR,7aS)-rel-
- CAS Number:174758-17-9
- Molecular formula:C7H12N2S
- Molecular Weight:156.25
Yield:174758-17-9 70%
Reaction Conditions:
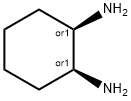
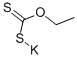
Stage #1: 1,2-diaminocyclohexane;potassium ethylxanthate in ethanol;lithium hydroxide monohydrate; for 4 h;Reflux;
Stage #2: with glacial acetic acid in lithium hydroxide monohydrate at 20; pH=3.5; for 0.5 h;
Steps:
General synthesis of 2H-benzo[d]imidazole-2-thione (Org. Synth., 1950, 30, 56).
General procedure: In a round-bottom flask were added 9.24 mmol of diamine and 1.7 g (11.09 mmol) of potassium ethyl xanthate in 30 mL of ethanol and 1 mL of water. The mixture was heated under reflux for 4h. After that, a small amount of activated charcoal was added to the reaction and kept under reflux for 30 minutes. The reaction was filtrated and acidified to a pH of 3.5 with a diluted solution of acetic acid in water. The solution was partially concentrated under reduced pressure when it was observed the precipitation of a white solid. It was added more 20 mL of water and the mixture was cooled using an ice bath. The precipitated was filtrated to obtain the pure 2Z7-benzo[7]imidazole-2-thiones.Synthesis of c/s-octahydro-2//-beiizo|d|iniidazole-2-thioneIt was obtained 1.02 g of the title compound as glistening yellow crystals (70% yield).
References:
WO2022/129256,2022,A1 Location in patent:Page/Page column 48