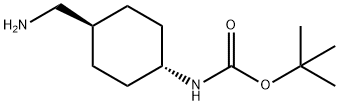
tert-Butyl (trans-4-(aminomethyl)cyclohexyl)carbamate synthesis
- Product Name:tert-Butyl (trans-4-(aminomethyl)cyclohexyl)carbamate
- CAS Number:177583-27-6
- Molecular formula:C12H24N2O2
- Molecular Weight:228.33
![Carbamic acid, N-[trans-4-(azidomethyl)-cyclohexyl]-, 1,1-dimethylethyl ester](/CAS/GIF/956352-36-6.gif)
956352-36-6
3 suppliers
inquiry

177583-27-6
84 suppliers
$31.00/100mg
Yield:-
Reaction Conditions:
with lithium hydroxide monohydrate;triphenylphosphine in tetrahydrofuran at 20 - 80; for 1.5 h;
Steps:
3.4
Azide obtained in Step 3 was dissolved in tetrahydrofuran (900 ml) at room temperature. Triphenylphosphine (103 g, 392 mmol) and water (90 ml) were sequentially added thereto and stirred at 80 °C for 1.5 hours. The solvent (770 ml) was removed and water (300 ml), ethyl acetate (400 ml) and 2N hydrochloric acid (150 ml) were sequentially added to become pH 2.5 and liquid-liquid extraction was carried out. The organic layer was extracted with 2N hydrochloric acid and the water layer was added thereto. The mixture was washed with ethyl acetate and 2N sodium hydroxide was added to alkalinize and repeatedly extracted with ethyl acetate and chloroform. The organic layer was added thereto and dried over magnesium sulfate anhydrous. The solvent was removed under reduced pressure and hexane was added to the residue. The precipitated crystals were collected with filtration and washed with hexane to give the desired amine (41.7 g, 53 % yield) (through Step 3 to 4). 1H-NMR (DMSO-d6) δppm: 0.77-0.96 (m, 2H), 1.00-1.18 (m, 3H), 1.37 (s, 9H), 1.67-1.82 (m, 4H), 2.30-2.38 (m, 2H), 2.90-3.60 (m, 2H), 3.05-3.22 (m, 1H), 6.66 (d, 1H, J = 7.2 Hz).
References:
EP2017261,2009,A1 Location in patent:Page/Page column 31-32
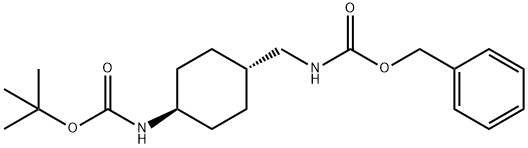
171846-13-2
4 suppliers
inquiry

177583-27-6
84 suppliers
$31.00/100mg
![Carbamic acid, [[4-[[(1,1-dimethylethoxy)carbonyl]amino]cyclohexyl]methyl]-, 2-propenyl ester, trans- (9CI)](/CAS/20210305/GIF/177583-52-7.gif)
177583-52-7
0 suppliers
inquiry

177583-27-6
84 suppliers
$31.00/100mg
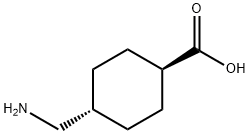
1197-18-8
921 suppliers
$10.00/5g

177583-27-6
84 suppliers
$31.00/100mg
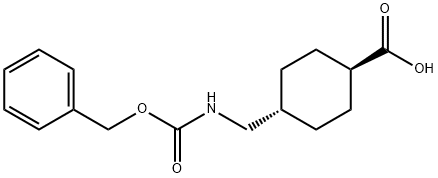
27687-12-3
12 suppliers
inquiry

177583-27-6
84 suppliers
$31.00/100mg