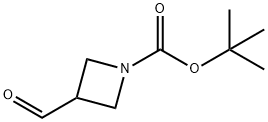
3-FORMYL-AZETIDINE-1-CARBOXYLIC ACID TERT-BUTYL ESTER synthesis
- Product Name:3-FORMYL-AZETIDINE-1-CARBOXYLIC ACID TERT-BUTYL ESTER
- CAS Number:177947-96-5
- Molecular formula:C9H15NO3
- Molecular Weight:185.22
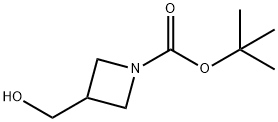
142253-56-3

177947-96-5
The general procedure for the synthesis of tert-butyl 3-formylazetidine-1-carboxylate from 1-Boc-3-hydroxymethylazetidine was as follows: to a stirred solution of oxalyl chloride (4.28 mL, 49.8 mmol) in dichloromethane (DCM, 5 mL) was added slowly and dropwise to dimethylsulfoxide (DMSO, 0.71 mL, 9.96 mmol) at -78 °C. The reaction mixture was continued to be stirred at this temperature for 15 minutes. Subsequently, tert-butyl 3-(hydroxymethyl)azetidine-1-carboxylate (0.69 g, 3.69 mmol) was added and triethylamine (2.1 mL, 14.75 mmol) was added immediately. The reaction system was gradually warmed to room temperature and diluted with DCM (30 mL). The reaction solution was washed with water and the organic phase was dried over anhydrous sodium sulfate (Na2SO4), filtered and concentrated under reduced pressure. The crude product was purified by column chromatography (using an Isolute column, eluent: heptane/ethyl acetate gradient, 0-100%) to give tert-butyl 3-formylazetidine-1-carboxylate (0.19 g, 27% yield).

142253-56-3
251 suppliers
$6.00/1g

177947-96-5
175 suppliers
$11.00/100mg
Yield:177947-96-5 99%
Reaction Conditions:
with 1-hydroxy-3H-benz[d][1,2]iodoxole-1,3-dione in ethyl acetateReflux;
Steps:
1 tert-butyl 3-formylazetidine-1-carboxylate (1.3)
To a stirringsolutionof tert-butyl 3-(hydroxymethyl)azetidine-1-carboxylate(20.96 g, 112 mmol) in EA (500 ml)was added IBX (62.69 g, 224 mmol) and the reaction was refluxed overnight. The reaction was cooledto rt, PE (500 ml)was added and the reaction mixture was filtered. The filtrate was concentrated under reduced pressureto give compound1.3 as yellow oil product (20.56 g,99%). 1H NMR (400 MHz, CDCI3) i5 1.44 (s, 9 H), 3.32-3.4015 (m, 1 H), 4.07-4.14 (m, 4 H), 9.85 (d, J = 2.0Hz, 3 H).
References:
ACHAOGEN, INC.;LINSELL, Martin Sheringham;AGGEN, James Bradley;DOZZO, Paola;HILDEBRANDT, Darin James;COHEN, Frederick;KASAR, Ramesh Annasaheb;KANE, Timothy Robert;GLIEDT, Micah James;MCENROE, Glenn A. WO2014/165075, 2014, A1 Location in patent:Page/Page column 45; 46
610791-05-4
158 suppliers
$5.00/250mg

177947-96-5
175 suppliers
$11.00/100mg
142253-54-1
176 suppliers
$9.00/250mg

177947-96-5
175 suppliers
$11.00/100mg
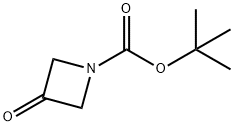
398489-26-4
441 suppliers
$9.00/5g
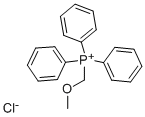
4009-98-7
365 suppliers
$5.00/10g

177947-96-5
175 suppliers
$11.00/100mg

142253-56-3
251 suppliers
$6.00/1g

177947-96-5
175 suppliers
$11.00/100mg