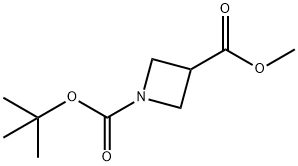
Methyl 1-Boc-azetidine-3-carboxylate synthesis
- Product Name:Methyl 1-Boc-azetidine-3-carboxylate
- CAS Number:610791-05-4
- Molecular formula:C10H17NO4
- Molecular Weight:215.25

67-56-1
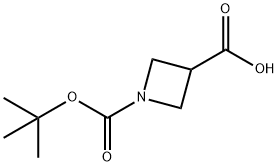
142253-55-2

610791-05-4
General procedure for the synthesis of methyl 1-(tert-butoxycarbonyl)azetidine-3-carboxylate from methanol and 1-Boc-azetidine-3-carboxylic acid: 1-(tert-butoxycarbonyl)azetidine-3-carboxylic acid (AXL016917, 1000 mg, 4.97 mmol) was dissolved in a mixture of solvents with methanol (5 mL) and toluene (20 mL), and subsequently the solution was cooled to 0°C. Trimethylsilyl diazomethane (7.45 mmol) was slowly added dropwise over a period of 15 min, during which slight bubble production was observed. The color of the solution gradually changed from clear to light yellow. The reaction mixture was continued to be stirred at 0 °C for 10 min, followed by slow warming to room temperature over a period of 30 min. Upon completion of the reaction, toluene was removed by concentration and distillation under reduced pressure to afford 1.055 g of methyl 1-(tert-butoxycarbonyl)azetidine-3-carboxylate (99% crude yield), which could be used in the next step of the reaction without further purification.

142253-55-2
334 suppliers
$6.00/1g

18107-18-1
210 suppliers
$33.00/5g

610791-05-4
158 suppliers
$5.00/250mg
Yield:610791-05-4 100%
Reaction Conditions:
in methanol;
Steps:
84.a
(a) 1-tert-Butyl 3-methyI azetidine-l,3-dicarboxylate; 1-(ter^Butoxycarbonyl)azetidine-3-carboxylic acid (2.42 g, 12.0 mmol) was dissolved in MeOH (30 mL) and TM5CHN2 (30.1 ml, 60.1 mmol) was added drop- wise at room temperature (reaction became warm and gas was evolved). TM5CHN2 was added until a persistent yellow color was produced indicating excess reagent. AcOH was added drop- wise to quench the excess TM5CHN2 and then the reaction mixture was concentrated under reduced pressure and azeotroped with toluene (3 x 20 mL) to remove any trace MeOH and AcOH. The crude material was used without any further purification assuming 100 % yield. 1H NMR (400 MHz, CDCl3): 6 1.44 (9H, s), 3.29-3.39 (1H, m), 3.75 (3H, s), 4.07-4.13 (4H, m).
References:
WO2006/73361,2006,A1 Location in patent:Page/Page column 171

67-56-1
790 suppliers
$9.00/25ml

142253-55-2
334 suppliers
$6.00/1g

610791-05-4
158 suppliers
$5.00/250mg

142253-55-2
334 suppliers
$6.00/1g

74-88-4
356 suppliers
$15.00/10g

610791-05-4
158 suppliers
$5.00/250mg

24424-99-5
868 suppliers
$13.50/25G
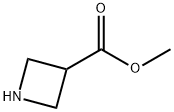
343238-58-4
52 suppliers
inquiry

610791-05-4
158 suppliers
$5.00/250mg
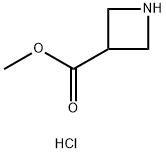
100202-39-9
204 suppliers
$7.00/1g

24424-99-5
868 suppliers
$13.50/25G

610791-05-4
158 suppliers
$5.00/250mg