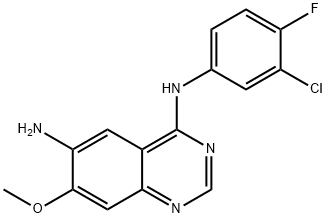
N-(3-chloro-4-fluorophenyl)-7-Methoxy-6-aminoquinazolin-4-aMine synthesis
- Product Name:N-(3-chloro-4-fluorophenyl)-7-Methoxy-6-aminoquinazolin-4-aMine
- CAS Number:179552-75-1
- Molecular formula:C15H12ClFN4O
- Molecular Weight:318.73
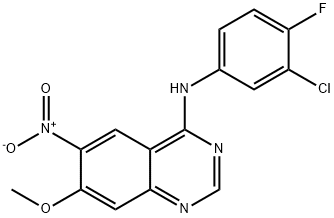
179552-74-0

179552-75-1
General procedure for the synthesis of N4-(3-chloro-4-fluorophenyl)-7-methoxy-6-nitroquinazolin-4-amine from N-(3-chloro-4-fluorophenyl)-7-methoxyquinazolin-4,6-diamine: N-(3-chloro-4-fluorophenyl)-7-methoxy-6-nitroquinazolin-4-amine (10 g, 28.6 mmol) was dissolved in ethanol (200 mL) and THF (100 mL) in a solvent mixture. To this solution, water (50 mL) and saturated ammonium chloride solution (50 mL) were sequentially added at room temperature, followed by iron powder (6.5 g, 116 mmol). The reaction mixture was heated to 80 °C and the reaction was stirred at this temperature for 3 hours. After completion of the reaction, the mixture was filtered through diatomaceous earth and the filter cake was washed with ethanol. Water (100 mL) was added to the filtrate and a yellow-white solid precipitated. The solid was filtered and dried to afford the target compound N4-(3-chloro-4-fluorophenyl)-7-methoxyquinazoline-4,6-diamine (8 g, 88% yield) as a yellow solid.1H-NMR (400 MHz, DMSO-d6): δ 9.41 (s, 1H), 8.38 (s, 1H), 8.20-8.17 (m, 1H), 7.82- 7.80 (m, 1H), 7.41-7.37 (m, 2H), 5.36 (s, 2H), 3.97 (s, 3H).

179552-74-0
117 suppliers
inquiry

179552-75-1
105 suppliers
$73.34/1g
Yield: 99%
Reaction Conditions:
with hydrogen;nickel in tetrahydrofuran
Steps:
10
(3-Chloro-4-fluoro-phenyl)-(7-fluoro-6-nitro-quinazolin-4-yl)-amine was suspended in 100 ml methanol and 2 ml 50% NaOH in water was added and the mixture was heated at 70° C. for 2 hours. The mixture was then poured into water and stirred vigorously for 30 minutes, then filtered and washed with water and dried under vacuum at 60° C. overnight to give 7.2 g of (3-Chloro-4-fluoro-phenyl)-(7-methoxy-6-nitro-quinazolin-4-yl)-amine. 7.1 g of (3-Chloro-4-fluoro-phenyl)-(7-methoxy-6-nitro-quinazolin-4-yl)-amine was reduced using Raney nickel catalyst in THF, then filtered and evaporated to give 6.4 g N4-(3-Chloro-4-fluoro-phenyl)-7-methoxy-quinazoline-4,6-diamine (99% yield). This product was reacted with 4-Chloro-but-2-enoyl chloride as described in Scheme 1 to provide 4-Chloro-but-2-enoic acid [4-(3-chloro-4-fluoro-phenylamino)-7-methoxy-quinazolin-6-yl]-amide. 300 g of 4-Chloro-but-2-enoic acid [4-(3-chloro4-fluoro-phenylamino)-7-methoxy-quinazolin-6-yl]-amide and 78 mg azepane were dissolved in 5 ml THF and purged with nitrogen. 0.25 ml DIEA was added and the mixture was stirred at 70° C. for 2 days. The mixture was then diluted with 20 ml ethyl acetate, washed with water and brine and dried over Na2SO4. The resulting solids were flash chromatographed with 0-4% methanol in chloroform. The product was dissolved in CH2Cl2 and treated with excess HCl and ether, then evaporated to dryness to give 115 mg of 4-Azepan-1-yl-but-2-enoic acid [4-(3-chloro-4-fluoro-phenylamino)-7-methoxy-quinazolin-6-yl]-amide (33% yield). (M+H)+ @484.
References:
Pfizer Inc US2005/250761, 2005, A1 Location in patent:Page/Page column 22
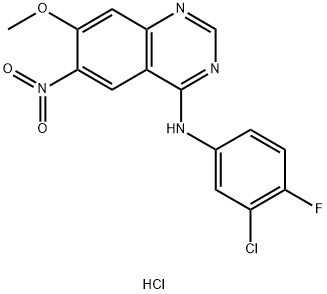
1012057-48-5
0 suppliers
inquiry

179552-75-1
105 suppliers
$73.34/1g
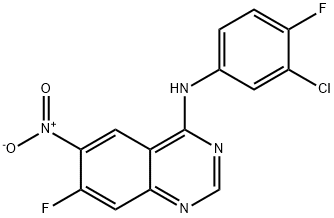
162012-67-1
333 suppliers
$8.00/1g

179552-75-1
105 suppliers
$73.34/1g
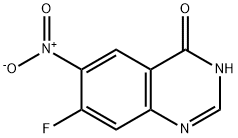
162012-69-3
367 suppliers
$6.00/250mg

179552-75-1
105 suppliers
$73.34/1g
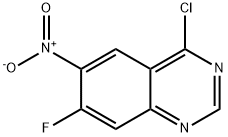
162012-70-6
147 suppliers
$125.00/25mg

179552-75-1
105 suppliers
$73.34/1g