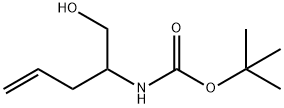
Carbamic acid, [1-(hydroxymethyl)-3-butenyl]-, 1,1-dimethylethyl ester (9CI) synthesis
- Product Name:Carbamic acid, [1-(hydroxymethyl)-3-butenyl]-, 1,1-dimethylethyl ester (9CI)
- CAS Number:183247-77-0
- Molecular formula:C10H19NO3
- Molecular Weight:201.26

24424-99-5
868 suppliers
$13.50/25G
1380005-74-2
16 suppliers
inquiry
![Carbamic acid, [1-(hydroxymethyl)-3-butenyl]-, 1,1-dimethylethyl ester (9CI)](/CAS/GIF/183247-77-0.gif)
183247-77-0
6 suppliers
inquiry
Yield:183247-77-0 78%
Reaction Conditions:
with sodium hydroxide in methanol;lithium hydroxide monohydrate at 10 - 30; pH=8 - 9; for 8 h;Large scale;
Steps:
1.3; 2.3
Tetrahydrofuran (250L) and compound 3 (50kg, 214.0mol, 1.0eq) were added to the 1500L reactor, N-methylmorphorine (26.0kg, 257.2mol, 1.2eq) was added to the reactor, and the nitrogen protection decreased to 0 ~ 10 °C. P-toluenesulfonyl chloride (44.8 kg, 235.1mol, 1.1eq) was dissolved in tetrahydrofuran (150L) and dripped at a temperature control of 0~10 °C. Drip, stir for 2h, then filter (solid discard), cool the filtrate to -10 ~ -5 °C. Sodium borohydride (12.2kg, 321.5mol, 1.5eq) was dissolved in 0.4% aqueous sodium hydroxide solution (100L), dripped into the reaction solution, and the temperature was controlled -10 ~ 0 °C. Drops, slowly rise to room temperature and stir for 1 h. The reaction solution was slowly quenched into concentrated hydrochloric acid (50L), and the tetrahydrofuran was concentrated under reduced pressure.Add concentrated hydrochloric acid (300L), rise to 95 ~ 105 °C reflux reaction for 12h, reduce to 0 ~ 10 °C, filter.The filter cake was added to water (400L), the di-tert-butyl dicarbonate (51.2kg, 235.1mol, 1.1eq) was dissolved in methanol (100L) and dripped, and the pH = 8-9 was adjusted with 20% sodium hydroxide, and the temperature was controlled by 10 ~ 30 °C. After drip, continue the reaction for 8 h. The reaction solution was concentrated under reduced pressure to remove methanol, extracted with dichloromethane (300L x3), washed with organic phase combined with water (300L), washed with 10% citric acid (300L), and washed with water (300L). The organic phase was concentrated to give compound 6 (33.6 kg, 166.9 mol), yield 78%, purity of 99.9% (ELSD detection). Compound 6 is (1-hydroxypent-4-en-2-yl) carbamic acid tert-butyl ester.
References:
CN114805134,2022,A Location in patent:Paragraph 0054; 0061-0065; 0066; 0073-0077