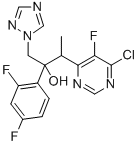
(2R,3S/2S,3R)-3-(4-Chloro-5-fluoro-6-pyrimidinyl)-2-(2,4-difluorophenyl)butan-2-ol hydrochloride synthesis
- Product Name:(2R,3S/2S,3R)-3-(4-Chloro-5-fluoro-6-pyrimidinyl)-2-(2,4-difluorophenyl)butan-2-ol hydrochloride
- CAS Number:188416-35-5
- Molecular formula:C16H13ClF3N5O
- Molecular Weight:383.76
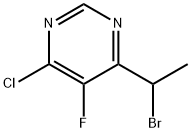
188416-28-6
178 suppliers
$100.00/1g
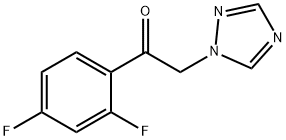
86404-63-9
417 suppliers
$19.00/25g

188416-35-5
191 suppliers
$145.00/1g
Yield:188416-35-5 87%
Reaction Conditions:
with lead;samarium diiodide;1-benzyl-3-(2-pyridylmethyl)-1H-benzimidazolium chloride;iodine;zinc in tetrahydrofuran at -5 - 40; for 1.33333 h;Inert atmosphere;
Steps:
1-2 Example 1
200 mesh Zn powder (25g, 385mmol), Pb powder (1g, 9.4mmol), SmI2 (4g, 9.9mmol), NHC-III (7g, 20.8mmol) and THF (150.0mL) were added to a 500mL three flask, Stir evenly at room temperature. The reaction flask was replaced with N2 for at least 3 times. Dissolve elemental iodine I2 (16g, 31.5mmol) in 50mL THF solution, add it into the reaction flask dropwise with a constant pressure dropping funnel, and control the reaction temperature at 30-40°C , Accelerating the drop acceleration), the remaining about 10mL I2 THF solution does not drop. Continue stirring at this temperature for 20 min. After 20 minutes, the temperature was lowered, and the reaction temperature was controlled to -5°C . The compound represented by formula I (26.8 g, 120 mmol) and the compound represented by formula II (34.4 g, 144 mmol) were dissolved in 100 mL of THF, added to a constant-pressure dropping funnel, and mixed with the remaining I2 THF solution before. Add the mixed drops into the reaction bottle, and control the reaction temperature to -5-0°C . Note that the dripping is uninterrupted during the dropping process. After the addition is complete, continue to stir at -5°C for 1h. After the reaction was completed, glacial acetic acid (20 mL) and water (80 mL) were quickly added dropwise to the reaction flask, and stirring was continued at 5-10°C for 30 min. After 30 minutes of filtration, the filtrate was collected and concentrated to dryness. Then add 200 mL of ethyl acetate and adjust the pH to about 1 with 2M HCl. Stand still and separate liquids. The organic layer was washed once with 2M HCl, and the aqueous layers were combined. The aqueous layer was extracted twice with ethyl acetate (2 × 10.00 mL), and the organic phases were combined. The organic phase was washed twice with an aqueous solution of EDTA at a concentration of 0.2 g / mL twice (50 mL / time), the aqueous layer was extracted twice with ethyl acetate (10.0 mL / time), and all organic layers were combined. The organic layer was washed with a saturated aqueous solution of sodium thiosulfate (10.0 mL × 3) until the starch potassium iodide test paper did not turn blue. After standing, the layers were separated, and the aqueous phase was extracted twice with ethyl acetate (10.0 mL / time). Floor. Several layers were dried over anhydrous magnesium sulfate, filtered, and then concentrated to about 100 mL, then 16 mL of isopropanol hydrogen chloride solution (7 mL / g) was added, and a solid precipitated immediately. Stir at 25 ° C for 2h, then place at 5 ° C and stir for about 10h. Filtration yielded product III as a light yellow solid. The yield was 87%.
References:
Huaihua University;Xiang Dexuan;Liu Cijie;Zhang Li;Liu Shasha;Zheng Lijuan;Lin Hongwei;Ouyang Yuejun;Li Yuanxiang;Zeng Shunqin;Tang Yan;Luo Fei CN110724130, 2020, A Location in patent:Paragraph 0049-0052; 0059-0062

86404-63-9
417 suppliers
$19.00/25g
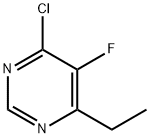
137234-74-3
339 suppliers
$10.00/1g

188416-35-5
191 suppliers
$145.00/1g
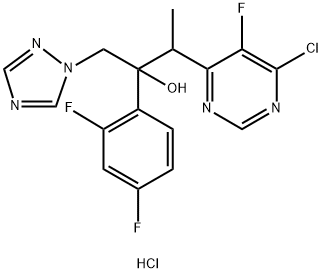
188416-20-8
109 suppliers
$393.00/1g

188416-35-5
191 suppliers
$145.00/1g

86404-63-9
417 suppliers
$19.00/25g

188416-35-5
191 suppliers
$145.00/1g
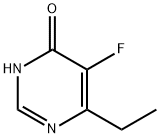
137234-87-8
233 suppliers
$9.00/1g

188416-35-5
191 suppliers
$145.00/1g