
1-(tert-butoxycarbonyl)-3-methylpiperidine-3,5-dicarboxylic acid synthesis
- Product Name:1-(tert-butoxycarbonyl)-3-methylpiperidine-3,5-dicarboxylic acid
- CAS Number:191544-71-5
- Molecular formula:C13H21NO6
- Molecular Weight:287.31
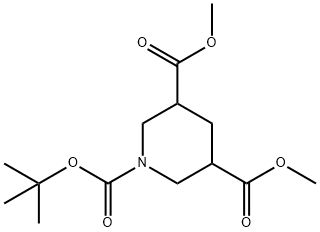
595555-70-7
18 suppliers
$432.00/1g

191544-71-5
7 suppliers
inquiry
Yield:-
Reaction Conditions:
Stage #1: 1-tert-butyl 3,5-dimethyl piperidine-1,3,5-tricarboxylatewith water;sodium hydroxide in methanol at 10 - 35; for 14 h;
Stage #2: with hydrogenchloride in water; pH=2;
Steps:
54
Reference Example 54 1-(tert-butoxycarbonyl)-5-(methoxycarbonyl)piperidine-3-carboxylic acid [Show Image] 1-tert-Butyl 3,5-dimethyl piperidine-1,3,5-tricarboxylate (75 g) was dissolved in methanol (375 ml), and 2 M aqueous sodium hydroxide solution (125 ml) was added dropwise at room temperature. The reaction mixture was stirred at room temperature for 14 hr, and methanol was evaporated under reduced pressure. The concentrated solution was diluted with saturated aqueous sodium hydrogen carbonate solution (100 ml) and washed twice with ethyl acetate. The basic aqueous layer was acidified (pH 2) with 6 M hydrochloric acid, and the mixture was extracted with ethyl acetate. The extract was dried over anhydrous magnesium sulfate, and the solvent was evaporated under reduced pressure to give the object compound (71 g). 1H-NMR (CDCl3) δ 1.33-1.50 (9H, m), 1.60-1.82 (1H, m), 1.96-2.22 (1H, m), 2.41-2.58 (2H, m), 2.62-2.91 (2H, m), 3.34-3.91 (1H, m), 3.71 (3H, s), 4.37 (1H, br s), 7.55-8.47 (1H, m).
References:
EP2202228,2010,A1 Location in patent:Page/Page column 137
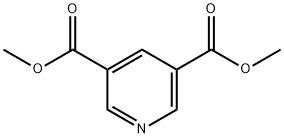
4591-55-3
116 suppliers
$57.00/2g

24424-99-5
871 suppliers
$13.50/25G

191544-71-5
7 suppliers
inquiry

595555-70-7
18 suppliers
$432.00/1g
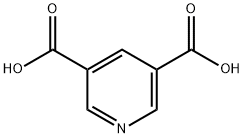
499-81-0
320 suppliers
$7.00/5g

191544-71-5
7 suppliers
inquiry

4591-55-3
116 suppliers
$57.00/2g

191544-71-5
7 suppliers
inquiry

24424-99-5
871 suppliers
$13.50/25G

191544-71-5
7 suppliers
inquiry