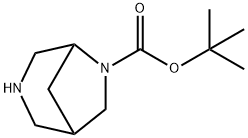
RaceMic tert-butyl 3,6-diazabicyclo[3.2.1]octane-6-carboxylate synthesis
- Product Name:RaceMic tert-butyl 3,6-diazabicyclo[3.2.1]octane-6-carboxylate
- CAS Number:194032-49-0
- Molecular formula:C11H20N2O2
- Molecular Weight:212.29
![Ethanamine, N-[(3,5-dichlorophenyl)methylene]-2,2-diethoxy-](/CAS/20210305/GIF/1000210-73-0.gif)
1000210-73-0
0 suppliers
inquiry
![RaceMic tert-butyl 3,6-diazabicyclo[3.2.1]octane-6-carboxylate](/CAS/GIF/194032-49-0.gif)
194032-49-0
48 suppliers
$150.00/50mg
Yield: 40%
Reaction Conditions:
with ammonia;copper(l) iodide in water at 100; for 16 h;
Steps:
5
The tert-butyl 2,4-bis(hydroxymethyl)pyrrolidine-1-carboxylate was dissolved in 300 mL of dry dichloromethane and cooled to 00C. Triethylamine (9.7 mL, 70 mmol) was added to the cooled solution, followed by a careful addition of methanesulfonyl chloride (5.4 mL, 70 mmol). The reaction was stirred at ambient temperature for 16 h. Saturated ammonium chloride solution (200 mL) was added, and the layers were separated. The aqueous layer was washed with dichloromethane (200 mL), and the combined organic layers were dried over anhydrous magnesium sulfate, filtered, and concentrated by evaporation of the volatiles. The residual oil, tert-butyl 2,4-bis((methylsulfonyloxy)methyl)pyrrolidine-1- carboxylate, was placed in 200 mL pressure tubes (-10 mmol maximum in each tube). Concentrated aqueous ammonium hydroxide (150 mL) and CuI (190 mg, 10 mol%) were added to each pressure tube. The tubes were sealed and heated at 1000C for 16 h. The tubes were cooled to ambient temperature, and the reaction mixture was concentrated by rotary evaporation at 600C (bath temperature). The solid was dissolved in methanol and filtered through diatomaceous earth to remove copper salts. The solvent was removed by rotary evaporation, and the residue was purified using an Analogix IntelliFlash 280 system with a SF25-120g Si column, eluting with a methanol in chloroform gradient (0-50% methanol over 30 min). Evaporation of the solvent gave tert-butyl 3,6- diazabicyclo[3.2.1]octane-6-carboxylate as a viscous oil (4.1 g, 40%).
References:
TARGACEPT, INC. WO2008/112734, 2008, A1 Location in patent:Page/Page column 69
![tert - butyl 3 - benzyl - 3,6 - diazabicyclo[3.2.1]octane - 6 - carboxylate](/CAS/20180808/GIF/848591-68-4.gif)
848591-68-4
12 suppliers
$455.00/1g
![RaceMic tert-butyl 3,6-diazabicyclo[3.2.1]octane-6-carboxylate](/CAS/GIF/194032-49-0.gif)
194032-49-0
48 suppliers
$150.00/50mg
![Tert-Butyl2-azabicyclo[2.2.1]hept-5-ene-2-carboxylate](/CAS/GIF/188345-71-3.gif)
188345-71-3
96 suppliers
$37.20/250mg
![RaceMic tert-butyl 3,6-diazabicyclo[3.2.1]octane-6-carboxylate](/CAS/GIF/194032-49-0.gif)
194032-49-0
48 suppliers
$150.00/50mg

24424-99-5
871 suppliers
$13.50/25G
![RaceMic tert-butyl 3,6-diazabicyclo[3.2.1]octane-6-carboxylate](/CAS/GIF/194032-49-0.gif)
194032-49-0
48 suppliers
$150.00/50mg
1058737-58-8
2 suppliers
inquiry
![RaceMic tert-butyl 3,6-diazabicyclo[3.2.1]octane-6-carboxylate](/CAS/GIF/194032-49-0.gif)
194032-49-0
48 suppliers
$150.00/50mg