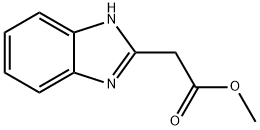
1H-Benzimidazole-2-aceticacid,methylester(9CI) synthesis
- Product Name:1H-Benzimidazole-2-aceticacid,methylester(9CI)
- CAS Number:49672-05-1
- Molecular formula:C10H10N2O2
- Molecular Weight:190.2

67-56-1
790 suppliers
$7.29/5ml-f
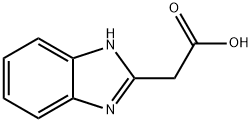
13570-08-6
114 suppliers
$47.00/1g

49672-05-1
29 suppliers
$58.50/5g
Yield: 86%
Reaction Conditions:
with thionyl chloride at 0 - 20; for 18 h;
Steps:
Methyl 2-(1H-1,3-benzodiazol-2-yl)acetate
Thionyl dichloride (0.7 ml, 9.65 mmol) was added dropwise to an ice-cooled (0°C) suspension of 2-(1H-1,3- benzodiazol-2-yl)acetic acid (179) (0.7 g, 3.97 mmol) in MeOH (30 ml). The reaction mixture was allowed to warm to room temperature and stirred for 18 h. The mixture was poured onto saturated NaHCQ (40 ml) and extracted with DCM (3 x 20 ml). The combined organic layers were washed with brine (30 ml), dried (NaS04), filtered and evaporated to give the title compound (0.65 g, 86%) as a cream solid. 1H-NMR(CDCI3, 500 MHz): d[ppm]= 10.10 (brs, 1H), 7.72 (brs, 1H), 7.46 (brs, 1H), 7.29-7.23 (m, 2H), 4.09 (s, 2H), 3.82 (s, 3H) HPLCMS (Method F): [m/z]: 191.2 [M+Hf
References:
VIFOR (INTERNATIONAL) AG;DÜRRENBERGER, Franz;BUHR, Wilm;BURCKHARDT, Susanna;BURGERT, Michael;KALOGERAKIS, Aris;REIM, Stefan;MANOLOVA, Vania;BOYCE, Susan;YARNOLD, Christopher John;PENA, Paula;SHEPHERD, Jon;LECCI, Cristina;JARJES-PIKE, Richard;SCOTT, John WO2017/68089, 2017, A2 Location in patent:Page/Page column 243

67-56-1
790 suppliers
$7.29/5ml-f
4857-04-9
207 suppliers
$6.00/1g
201230-82-2
1 suppliers
inquiry

49672-05-1
29 suppliers
$58.50/5g
4414-88-4
232 suppliers
$8.00/5g

49672-05-1
29 suppliers
$58.50/5g