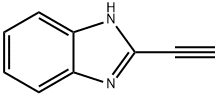
1H-Benzimidazole,2-ethynyl-(9CI) synthesis
- Product Name:1H-Benzimidazole,2-ethynyl-(9CI)
- CAS Number:53243-15-5
- Molecular formula:C9H6N2
- Molecular Weight:142.16
![1H-Benzimidazole, 2-[2-[tris(1-methylethyl)silyl]ethynyl]-](/CAS/20210305/GIF/486999-92-2.gif)
486999-92-2
0 suppliers
inquiry

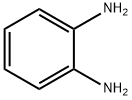
53243-15-5
10 suppliers
inquiry
Yield:53243-15-5 97%
Reaction Conditions:
with tetrabutyl ammonium fluoride in tetrahydrofuran at -40 - 0;
Steps:
Methyl l-(2-cyanoethyl)-3-(4-fluorophenethyl)-l//-indole-5-carboxylate (129).
The synthesis of 129 followed general procedures B and D to obtain the desired product as white solid (43% yield over two steps) using the Wittig salt, (4-fluorobenzyl) triphenylphosphonium bromide. 1 H- NMR (CDCh, 300 MHz) d 8.31 (s, 1H), 7.94 (d, 7 = 8.4 Hz, 1H), 7.27 (d, 7 = 8.7 Hz, 1H), 7.17 - 7.05 (m, 2H), 7.05 - 7.01 (m, 2H), 6.92 (s, 1H), 4.35 (t, 7 = 6.3 Hz, 2H), 3.93 (s, 3H), 3.10 - 2.89 (m, 4H), 2.76 (t, 7 = 6.6 Hz, 2H). 13C-NMR (CDCh, 75 MHz) d 168.20, 163.18, 159.96, 138.48, 137.67, (0404) 137.63, 130.16, 130.05, 128.20, 126.24, 123.86, 122.60, 121.88, 117.88, 117.38, 115.40, 115.12, (0405) 108.63, 52.19, 42.20, 35.76, 27.22, 19.42. MS (ESI) m/z = 351.2 [M+H]+.
References:
WO2019/191410,2019,A1 Location in patent:Page/Page column 54; 58
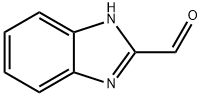
3314-30-5
180 suppliers
$21.21/500mgs:
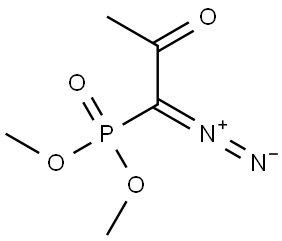
90965-06-3
212 suppliers
$30.25/1g

53243-15-5
10 suppliers
inquiry
54624-57-6
166 suppliers
$11.00/250mg

53243-15-5
10 suppliers
inquiry