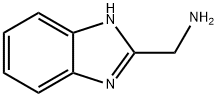
(1H-BENZO[D]IMIDAZOL-2-YL)METHANAMINE synthesis
- Product Name:(1H-BENZO[D]IMIDAZOL-2-YL)METHANAMINE
- CAS Number:5805-57-2
- Molecular formula:C8H9N3
- Molecular Weight:147.18
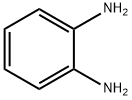
95-54-5
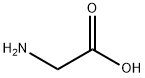
56-40-6
![(1H-BENZO[D]IMIDAZOL-2-YL)METHANAMINE](/CAS/GIF/5805-57-2.gif)
5805-57-2
GENERAL METHOD: O-phenylenediamine (0.54 g, 5 mmol) and glycine (0.53 g, 7 mmol) were sequentially added to a rapid flask containing 10 mL of toluene. The reaction mixture was heated and refluxed under magnetic stirring at 85-95 °C for 9 h. A colored solution was generated during the reaction. Upon completion of the reaction, the mixture was cooled to room temperature and allowed to stand overnight. The precipitated crystals were filtered and dried in air to give (1H-benzo[d]imidazol-2-yl)methylamine (10a) in 97.3% yield. In view of the high efficiency demonstrated by method B in the synthesis of 10a, the method was employed to synthesize the remaining compounds (10b-i). The NMR hydrogen spectrum (400 MHz, DMSO-d6) δ of the product 10a: 7.95 (s, 1H, NH), 7.82-7.80 (d, J = 8.03 Hz, 2H, Ar-H), 7.17-7.13 (m, 2H, Ar-H), 6.44-6.42 (t, J = 5.78 Hz, 2H, NH2-CH2). 3.55-3.53 (t, J = 5.78 Hz, 2H, CH2-NH2). NMR carbon spectrum (100 MHz, DMSO-d6) δ: 142.6, 139.3, 138.9, 125.3, 125.3, 119.2, 119.2, 41.7 ppm. uv-vis spectra (λmax, nm) (logεmax): 236 (1.7782), 290 (1.324), 407 ( 0.8541). IR spectra (KBr, cm-1): 3384, 3363 (NH of NH2, double peak), 3245 (NH), 3021 (aromatic CH), 2930 (aliphatic CH), 1605 (C=C), 1580 (C=N), 741 (Ar-H). Mass spectra (m/z, relative %): 295.04 (2M+1, 1.5%), 148.01 (MH, 24.5%), 147.03 (M+, 20%), 132.04 (M-NH, 100%), 118.07 (M-CH2=NH, 35.3%), 76.11 (68.3%).
Yield: 97.3%
Reaction Conditions:
in toluene at 85 - 95; for 9 h;Solvent;Reagent/catalyst;
Steps:
Method B
General procedure: o-Phenylenediamine (0.54 g, 5 mmol) and required amino acid or anthranilic acid (7 mmol) was added sequentially to 10 ml of toluene in a quickfit flask. The reacting mixture was heated under reflux at carefully controlled temperature of 85 - 95 oC for 9 h under the influence of a magnetic stirrer to obtain coloured solution which was allowed to cool overnight. The crystals formed was filtered and air-dried to afford (1H-benzo[d]imidazol-2-yl)methanamine, 10a in 97.3% yield. Due to high efficiency observed in Method B for the synthesis of 10a,it was therefore adopted as the viable method for the synthesis of the rest of the compounds 10b-i. Synthesis of (1H-benzo[d] imidazol- 2- yl) methanamine, 10aTreatment ofo-phenylenediamine (0.54 g, 5 mmol) with L-glycine (0.53 g, 7 mmol) afforded(1H-benzo[d]imidazol-2-yl)methanamine, 10a.1HNMR (400 MHz, DMSO-d6) δ: 7.95 (s, 1H, NH), 7.82-7.80 (d, J= 8.03 Hz, 2H, Ar-H), 7.17-7.13 (m, 2H, Ar-H), 6.44-6.42 (t, J= 5.78 Hz, 2H, NH2-CH2), 3.55-3.53 (t, J= 5.78 Hz, 2H, CH2-NH2). 13C-NMR (100 MHz, DMSO-d6) δ: 142.6, 139.3, 138.9, 125.3, 125.3, 119.2, 119.2, 41.7 ppm. lmax in nm (log εmax): 236 (1.7782), 290 (1.324), 407 (0.8541). IR (KBr): 3384, 3363 (N-H of NH2, two bands), 3245 (N-H), 3021 (C-H aromatic), 2930 (C-H aliphatic), 1605 (C=C), 1580 (C=N), 741 (Ar-H) cm-1. MS: in m/z (rel. %): 295.04 (2M + 1, 10.5%), 148.01 (MH, 24.5%), 147.03 (M+, 20%), 132.04 (M - NH, 100%), 118.07 (M - CH2=NH, 35.3%), 76.11 (68.3%).
References:
Ajani, Olayinka O.;Aderohunmu, Damilola V.;Olorunshola, Shade J.;Ikpo, Chinwe O.;Olanrewaju, Ifedolapo O. [Oriental Journal of Chemistry,2016,vol. 32,# 1,p. 109 - 120]
![tert-Butyl ((1H-benzo[d]imidazol-2-yl)methyl)carbamate](/CAS/GIF/189560-83-6.gif)
189560-83-6
21 suppliers
inquiry
![(1H-BENZO[D]IMIDAZOL-2-YL)METHANAMINE](/CAS/GIF/5805-57-2.gif)
5805-57-2
95 suppliers
$13.00/500mg
5993-91-9
88 suppliers
$59.39/5g
![(1H-BENZO[D]IMIDAZOL-2-YL)METHANAMINE](/CAS/GIF/5805-57-2.gif)
5805-57-2
95 suppliers
$13.00/500mg

95-54-5
543 suppliers
$9.00/1g
![(1H-BENZO[D]IMIDAZOL-2-YL)METHANAMINE](/CAS/GIF/5805-57-2.gif)
5805-57-2
95 suppliers
$13.00/500mg
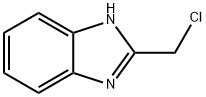
4857-04-9
207 suppliers
$6.00/1g
![(1H-BENZO[D]IMIDAZOL-2-YL)METHANAMINE](/CAS/GIF/5805-57-2.gif)
5805-57-2
95 suppliers
$13.00/500mg