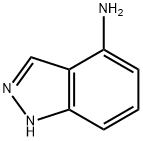
1H-INDAZOL-4-AMINE synthesis
- Product Name:1H-INDAZOL-4-AMINE
- CAS Number:41748-71-4
- Molecular formula:C7H7N3
- Molecular Weight:133.15
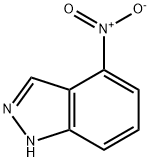
2942-40-7

41748-71-4
The general procedure for the synthesis of 4-aminoindazole from 4-nitroindazole is as follows: 1. 2-Methyl-3-nitroaniline (2.27 g, 14.91 mmol) was dissolved in acetic acid (60 mL) and an aqueous solution of sodium nitrite (1.13 g, 1.1 eq.) was added (5 mL). After 2 hours of reaction, the dark red solution was poured into ice/water and the precipitate was collected by filtration to give 4-nitro-1H-indazole (1.98 g, 81% yield). 2. 4-Nitro-1H-indazole (760 mg, 4.68 mmol), charcoal-loaded palladium (10%, catalyst), and ethanol (30 mL) were stirred under a hydrogen balloon for 4 hours. Upon completion of the reaction, the solvent was removed in vacuum by filtration through diatomaceous earth to afford 1H-indazol-4-ylamine (631 mg, 100% yield). 3. A suspension of 1H-indazol-4-ylamine (63 mg, 4.74 mmol) in 6 M hydrochloric acid (7.2 mL) was added dropwise to an aqueous solution (2 mL) of sodium nitrite (337 mg, 4.89 mmol) at below 0 °C. After stirring for 30 min, sodium tetrafluoroborate (724 mg) was added. The reaction mixture formed a viscous solution, which was filtered and washed briefly with water to give 1H-indazole-4-diazotetrafluoroborate (218 mg, 20% yield) as a dark red solid. 4. Dry methanol (4 mL) was purged with argon for 5 min and 1H-indazole-4-diazotetrafluoroborate (218 mg, 0.94 mmol), bis(pinacolato)diboron (239 mg, 1.0 eq.), and [1,1'-bis(diphenylphosphino)ferrocene]palladium(II) chloride (20 mg) were added. The reaction mixture was stirred for 5 hours and then filtered through diatomaceous earth. The residue was purified by fast chromatography to afford the target product 4-aminoindazole (117 mg).

2942-40-7
212 suppliers
$5.00/250mg

41748-71-4
144 suppliers
$19.00/250mg
Yield: 100%
Reaction Conditions:
with hydrogen;palladium 10% on activated carbon in ethanol for 4 h;
Steps:
5
Reference Example 5: Indazole-4-Boronate Ester (70): Route 1 EPO
References:
PIRAMED LIMITED WO2006/46031, 2006, A1 Location in patent:Page/Page column 31-33
![Ethanamine, N-[(3,5-dichlorophenyl)methylene]-2,2-diethoxy-](/CAS/20210305/GIF/1000210-73-0.gif)
1000210-73-0
0 suppliers
inquiry

41748-71-4
144 suppliers
$19.00/250mg
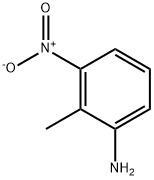
603-83-8
309 suppliers
$11.00/5g

41748-71-4
144 suppliers
$19.00/250mg
![Ethanamine, N-[(3,5-dichlorophenyl)methylene]-2,2-diethoxy-](/CAS/20210305/GIF/1000210-73-0.gif)
1000210-73-0
0 suppliers
inquiry
77894-69-0
73 suppliers
$29.00/100mg
![Ethanamine, N-[(3,5-dichlorophenyl)methylene]-2,2-diethoxy-](/CAS/20210305/GIF/1000210-73-0.gif)
1000210-73-0
0 suppliers
inquiry

41748-71-4
144 suppliers
$19.00/250mg
![Ethanamine, N-[(3,5-dichlorophenyl)methylene]-2,2-diethoxy-](/CAS/20210305/GIF/1000210-73-0.gif)
1000210-73-0
0 suppliers
inquiry

77894-69-0
73 suppliers
$29.00/100mg
![Ethanamine, N-[(3,5-dichlorophenyl)methylene]-2,2-diethoxy-](/CAS/20210305/GIF/1000210-73-0.gif)
1000210-73-0
0 suppliers
inquiry

41748-71-4
144 suppliers
$19.00/250mg