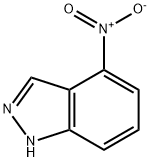
4-Nitro-1H-indazole synthesis
- Product Name:4-Nitro-1H-indazole
- CAS Number:2942-40-7
- Molecular formula:C7H5N3O2
- Molecular Weight:163.13
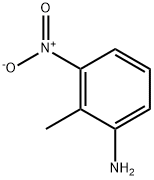
603-83-8

2942-40-7
General procedure for the synthesis of 4-nitroindazole from 2-methyl-3-nitroaniline: A. Preparation of 4-nitroindazole An aqueous sodium nitrite solution was prepared by dissolving sodium nitrite (20 g, 0.29 mol) in 50 mL of water. This aqueous sodium nitrite solution was added at once to a glacial acetic acid solution containing 2-methyl-3-nitroaniline (20 g, 0.13 mol) at 0 °C. The reaction system was vigorously stirred using an overhead stirrer. Immediately after the addition of sodium nitrite solution, precipitation generation was observed. Subsequently, the reaction mixture was gradually warmed up to room temperature and stirring was continued overnight. Upon completion of the reaction, the precipitate was separated by filtration and the filtrate was concentrated under vacuum. The resulting dark orange solid was suspended in water, filtered again and dried to give 14-21 g of dark orange solid product in 99% yield.

603-83-8
310 suppliers
$11.00/5g

2942-40-7
212 suppliers
$5.00/250mg
Yield:2942-40-7 99%
Reaction Conditions:
with sodium nitrite in water;acetic acid
Steps:
1.A A.
A. Preparation of 4-Nitroindazole from 2-Methyl-3-Nitro-Aniline Sodium nitrite (20 grams, 0.29 mol) was dissolved in 50 mL water. This solution was added all at once to 2-methyl-3-nitroaniline (20 grams, 0.13 moles) in glacial acetic acid near zero degrees C. The reaction was stirred vigorously with an overhead stirrer. An immediate precipitate occurred upon addition of sodium nitrite solution. The reaction was allowed to reach room temperature and stirred overnight. The precipitate was filtered off and the filtrate was concentrated in vacuo. The dark orange solid was suspended in water, filtered, and dried yielding 14-21 grams of a dark orange solid (99% yield).
References:
Eli Lilly and Company US6534504, 2003, B1