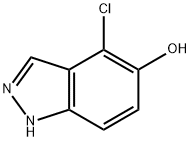
1H-Indazol-5-ol, 4-chloro- synthesis
- Product Name:1H-Indazol-5-ol, 4-chloro-
- CAS Number:478834-25-2
- Molecular formula:C7H5ClN2O
- Molecular Weight:168.58
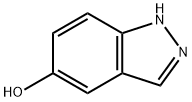
15579-15-4

478834-25-2
At room temperature, 1H-indazol-5-ol (1.60 g, 11.9 mmol) was dissolved in tetrahydrofuran (50 mL) and N-chlorosuccinimide (1.59 g, 11.9 mmol) was added. After 1 hour of reaction, the mixture was warmed up to 40 °C to continue the reaction for 2 hours, followed by warming up to 50 °C for 5 hours. Upon completion of the reaction, the reaction solution was poured into water (100 mL) and extracted with ethyl acetate (3 × 100 mL). The organic layers were combined, dried with anhydrous magnesium sulfate and concentrated under reduced pressure. The resulting residue was purified by silica gel column chromatography (eluent: hexane/ethyl acetate) to afford 4-chloro-5-hydroxy-1H-indazole (1.7365 g, 86% yield).1H-NMR (DMSO-d6) δ: 7.09 (1H, d, J = 8.8 Hz), 7.33 (1H, d, J = 8.8 Hz), 7.90 (1H, s) 9.71 (1H, s), 13.10 (1H, s).

15579-15-4
204 suppliers
$5.00/250mg

478834-25-2
52 suppliers
$110.00/10mg
Yield:478834-25-2 86%
Reaction Conditions:
with N-chloro-succinimide in tetrahydrofuran at 20 - 50; for 8 h;
Steps:
468 Example 468; Synthesis of 4-chloro-1H-indazol-5-ol
To a solution of the 1H-indazol-5-ol (1.60 g, 0.0119 mol) obtained in Reference Example 4 in tetrahydrofuran (50 ml) was added N-chlorosuccinimide (1.59 g, 0.0119 mol) at room temperature. After 1 hour, the mixture thus obtained was heated to 40°C, and after another 2 hour, the mixture was heated to 50°C. After 5 hours, the reaction solution was poured into water (100 ml) and extracted with ethyl acetate (100 ml x 3). The organic layer was dried over anhydrous magnesium sulfate and concentrated under reduced pressure, and the resulting residue was purified by a silica gel column chromatography (eluent: hexane/ethyl acetate) to obtain 4-chloro-1H-indazol-5-ol (1.7365 g, 86%).1H-NMR (DMSO-d6) δ; 7.09 (1H, d, J=8.8Hz), 7.33 (1H, d, J=8.8Hz), 7.90 (1H, s), 9.71 (1H, s), 13.10 (1H, s).
References:
EP1403255,2004,A1 Location in patent:Page 120
94444-96-9
134 suppliers
$24.00/250mg

478834-25-2
52 suppliers
$110.00/10mg
102-50-1
239 suppliers
$6.00/5g

478834-25-2
52 suppliers
$110.00/10mg