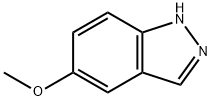
5-METHOXY-1H-INDAZOLE synthesis
- Product Name:5-METHOXY-1H-INDAZOLE
- CAS Number:94444-96-9
- Molecular formula:C8H8N2O
- Molecular Weight:148.16
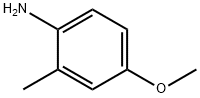
102-50-1

94444-96-9
General procedure for the synthesis of 5-methoxy-1H-indazole using 2-methyl-4-methoxyaniline as starting material: aqueous (8.1 ml) solution of sodium nitrite (3.38 g, 49.0 mmol) was slowly added dropwise to acetic acid (350 ml) solution of 4-methoxy-2-methylaniline (6.69 g, 48.8 mmol) under ice-water bath conditions. The temperature was strictly controlled not to exceed 25°C during the reaction, followed by continuous stirring at room temperature overnight. After completion of the reaction, the reaction mixture was poured into water and extracted with chloroform. The organic layers were combined, washed with saturated aqueous sodium chloride solution and dried over anhydrous magnesium sulfate. The solvent was removed by distillation under reduced pressure and the resulting crude product was purified by silica gel column chromatography (eluent ratio of chloroform/methanol=9/1) to give 5-methoxy-1H-indazole (1.30 g, 18% yield). The product was characterized by 1H-NMR (DMSO-d6) and the chemical shifts δ were 3.76 (3H, s), 6.98 (1H, dd, J=8.8,1.8 Hz), 7.15 (1H, d, J=1.8 Hz), 7.42 (1H, d, J=8.8 Hz), 7.93 (1H, s), and 12.89 (1H, brs).

102-50-1
239 suppliers
$6.00/5g

94444-96-9
134 suppliers
$24.00/250mg
Yield: 18%
Reaction Conditions:
with acetic acid;sodium nitrite in water at 20;
Steps:
4.a Reference Example 4; Synthesis of 1H-indazol-5-ol; (a) Synthesis of 5-methoxy-1H-indazole
A solution of sodium nitrite (3.38 g, 49.0 mmol) in water (8.1 ml) was added to a solution of 4-methoxy-2-methylaniline (6.69 g, 48.8 mmol) in acetic acid (350 ml) in an ice-water bath while maintaining the temperature at 25°C or lower, and stirred overnight at room temperature. Then, the reaction solution was poured into water and extracted with chloroform. The organic layer was washed with a saturated aqueous sodium chloride solution, dried over anhydrous magnesium sulfate and then distilled under reduced pressure to remove the solvent, and the resulting residue was purified by a silica gel column chromatography (eluent: chloroform/methanol = 9/1) to obtain 5-methoxy-1H-indazole (1.30 g, 18%).1H-NMR (DMSO-d6) δ; 3.76 (3H, s), 6.98 (1H, dd, J=8.8, 1.8Hz), 7.15 (1H, d, J=1.8Hz), 7.42 (1H, d, J=8.8Hz), 7.93 (1H, s), 12.89 (1H, brs).
References:
Sumitomo Pharmaceuticals Company, Limited EP1403255, 2004, A1 Location in patent:Page 44
31601-41-9
50 suppliers
$155.00/10g

94444-96-9
134 suppliers
$24.00/250mg
672-13-9
277 suppliers
$29.19/1g

94444-96-9
134 suppliers
$24.00/250mg
105728-90-3
179 suppliers
$12.00/1g

94444-96-9
134 suppliers
$24.00/250mg
459-60-9
363 suppliers
$7.00/5g

94444-96-9
134 suppliers
$24.00/250mg