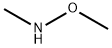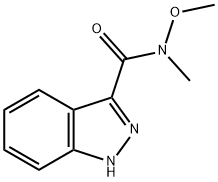
1H-INDAZOLE-3-(N-METHOXY-METHYL)CARBAMIDE synthesis
- Product Name:1H-INDAZOLE-3-(N-METHOXY-METHYL)CARBAMIDE
- CAS Number:351457-12-0
- Molecular formula:C10H11N3O2
- Molecular Weight:205.21
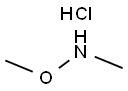
6638-79-5
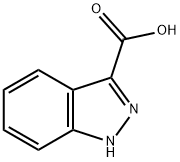
4498-67-3

351457-12-0
Indazole-3-carboxylic acid (10 g, 61.7 mmol) was used as a raw material, which was dissolved in DMF (100 mL), and carbonyldiimidazole (11 g, 67.84 mmol) was slowly added at room temperature, and gas was observed to be released during the reaction for 15 min. Subsequently, the reaction mixture was heated to 65 °C and maintained for 2 hours. After completion of the reaction, the mixture was cooled to room temperature, N,O-dimethylhydroxylamine hydrochloride (4.14 g, 67.8 mmol) was added and the mixture was again heated to 65 °C and reacted overnight. At the end of the reaction, the reaction mixture was cooled, the reaction was quenched with water, extracted with dichloromethane (CH2Cl2) and the organic phase was washed with water. The organic phases were combined and concentrated after drying to afford the target product N-methoxy-N-methyl-1H-indazole-3-carboxamide (10.3 g, 81.4% yield).

6638-79-5
589 suppliers
$6.00/25g

4498-67-3
486 suppliers
$6.00/5g

351457-12-0
64 suppliers
$30.00/1 g
Yield: 85%
Reaction Conditions:
Stage #1:N,O-dimethylhydroxylamine*hydrochloride;1H-Indazole-3-carboxylic acid with pyridine in tetrahydrofuran at 20; for 3 h;Cooling with ice;
Stage #2: with pyridine;1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride in tetrahydrofuran at 20;
Steps:
N-Methoxy-N-methyl-1H-indazole-3-carboxamide (6a)
To a solution of 1H-indazole-3-carboxylic acid (5, 4.87 g,30.0 mmol) in THF (250 mL) was added N,O-dimethylhydroxylaminehydrochloride (3.22 g, 33.0 mmol) at room temperature. Thereaction mixture was cooled on an ice-salt bath for 30 min, andpyridine (5.34 mL, 66.0 mmol) was added. The resultant reactionmixture was stirred on the ice-salt bath for 2 h and at room temperaturefor 1 h. Pyridine (5.34 mL, 66.0 mmol) and EDCHCl (11.5 g,60.0 mmol) were added. The reaction mixture was stirred at roomtemperature overnight and concentrated under reduced pressure,and the residue was triturated with H2O (500 mL). The solids werefiltered and washed with H2O (400 mL) to give 6a as a white solid(5.26 g, 85%). 1H NMR (CDCl3) d 3.57 (s, 3H, NCH3), 3.81 (s, 3H,OCH3), 7.24e7.28 (m, 1H, indazolyl-H), 7.39e7.43 (m, 1H, indazolyl-H), 7.55 (d, 1H, J 8.5, indazolyl-H), 8.21 (d, 1H, J 8.0, indazolyl-H),11.24 (br s, 1H, indazolyl-NH). HRMS (ESI-TOF) m/z 206.0927[MH]; calcd. for C10H12N3O2 206.0924 [MH].
References:
Long, Yi;Yu, Mingfeng;Ochnik, Aleksandra M.;Karanjia, Jasmine D.;Basnet, Sunita KC.;Kebede, Alemwork A.;Kou, Lianmeng;Wang, Shudong [European Journal of Medicinal Chemistry,2021,vol. 213,art. no. 113215]
91-56-5
474 suppliers
$5.00/25g

351457-12-0
64 suppliers
$30.00/1 g

4498-67-3
486 suppliers
$6.00/5g

351457-12-0
64 suppliers
$30.00/1 g
62-53-3
688 suppliers
$10.00/1g

351457-12-0
64 suppliers
$30.00/1 g