
1H-Indol-5-amine, 7-fluoro- synthesis
- Product Name:1H-Indol-5-amine, 7-fluoro-
- CAS Number:926028-84-4
- Molecular formula:C8H7FN2
- Molecular Weight:150.15

926028-83-3

926028-84-4
The general procedure for the synthesis of 7-fluoro-1H-indol-5-amine from 7-fluoro-5-aminoindoline was as follows: first, a mixture of 7-fluoro-1H-indole (18.5 g, 0.14 mol), borane-trimethylamine complex (80 g, 1.1 mol), and 1,4-dioxane (700 mL) was added to an aqueous solution of 37% HCl (80 mL) over 15 minutes . The temperature of the reaction solution was raised to 40°C, followed by stirring at room temperature for 16 hours. Next, the mixture was refluxed and boiled for 1 h. 6 M aqueous HCl solution (500 mL) was added and refluxing was continued for 15 min. The reaction solution was concentrated at atmospheric pressure and poured into an ice brine mixture. The aqueous phase was adjusted to alkaline with 25% ammonia and extracted with ethyl acetate. The organic phases were combined, dried over MgSO4, filtered and concentrated in vacuum. The residue was dissolved in a mixture of triethylamine (38 mL, 0.27 mol) and tetrahydrofuran (350 mL), cooled to 10 °C, acetyl chloride (11.2 g, 0.14 mol) was added, filtered and concentrated. Purification by rapid chromatography (ethyl acetate/heptane 50:50) afforded 1-(7-fluoro-2,3-dihydro-1H-indol-1-yl)ethanone (16.7 g, 0.09 mol). The product was dissolved in acetic acid (250 mL) and 100% nitric acid (5.8 mL, 0.14 mol) was added over 5 min and stirred for 2 h at room temperature. Another 6 mL of 100% nitric acid was added and stirring continued for 16 h. The mixture was poured into an ice brine mixture. It was adjusted to alkaline with 25% ammonia, extracted with ethyl acetate, the organic phases were combined, washed with brine, dried over MgSO4, filtered and concentrated in vacuum. The residue was crystallized with a mixture of ethyl acetate and propanol to give 1-(7-fluoro-5-nitro-2,3-dihydro-1H-indol-1-yl)ethanone (15.9 g). This product was dissolved in methanol (500 mL), ammonium formate (44.4 g, 0.7 mol) and 5 wt% palladium/activated charcoal (4.0 g) were added and refluxed for 30 min. It was cooled, filtered and concentrated in vacuum. The residue was dissolved in methanol (100 mL) and ethyl acetate (500 mL) and filtered to remove the precipitated ammonium formate. The mother liquor was concentrated and purified by fast chromatography (ethyl acetate/heptane 65:35) to afford 1-(5-amino-7-fluoro-2,3-dihydro-1H-indol-1-yl)ethanone (13.1 g, >91%). This compound was dissolved in methanol (350 mL), 28% aqueous sodium hydroxide solution (100 mL) and water (100 mL) and refluxed for 4 hours. Concentrated to about 200mL, brine (1L) was added and extracted with a mixture of ethyl acetate and tetrahydrofuran. The organic phases were combined, washed with brine, dried over MgSO4, filtered and concentrated in vacuum to give 7-fluoro-2,3-dihydro-1H-indol-5-amine (11.0 g, 96%). This compound was dissolved in p-xylene (500 mL), 5 wt% palladium/activated carbon (7.5 g) was added, and refluxed for 1.5 hr using a Dean-Stark splitter. After cooling and filtration, the filter cake was washed with ethyl acetate and tetrahydrofuran, the organic phases were combined and concentrated in vacuum. Purification by fast chromatography (ethyl acetate/heptane 50:50) afforded 7-fluoro-1H-indol-5-amine (3.3 g, 29%). Another batch (0.2 g) was prepared and combined. N-benzyliminodiacetic acid (5.9 g, 0.027 mol), 1,1'-carbonyldiimidazole (9.0 g, 0.056 mol) and tetrahydrofuran (175 mL) were refluxed for 30 min. A solution of 7-fluoro-1H-indol-5-amine (3.47 g, 0.023 mol) in tetrahydrofuran (75 mL) was added over 1 h. The solution was refluxed for 3 h and concentrated to 50 mL. Purification by rapid chromatography (ethyl acetate/heptane 80:20) afforded 4-benzyl-1-(7-fluoro-1H-indol-5-yl)piperazine-2,6-dione (7.8 g, 95% ). This product was dissolved in tetrahydrofuran (75 mL) and added dropwise to tetrahydrofuran over 60 min at 5-10 °C. It was stirred at 7 °C for 30 min and quenched by the addition of water (6.5 mL), 15% aqueous sodium hydroxide solution (3.25 mL) and water (16 mL). MgSO4 was added, filtered and concentrated in vacuum. Purification by rapid chromatography (ethyl acetate/heptane 50:50) afforded 5-(4-benzylpiperazin-1-yl)-7-fluoro-1H-indole (4.9 g, 63%).
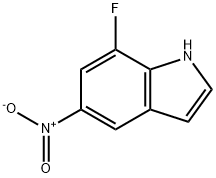
1167055-33-5
21 suppliers
inquiry

926028-84-4
34 suppliers
$45.00/10mg
Yield:926028-84-4 200 mg
Reaction Conditions:
with iron;ammonium chloride in water;ethyl acetate at 60; for 4 h;
Steps:
78.f (f) 7-Fluoro-1 H-indol-5-amine
To a stirred solution of 7-fluoro-5-nitro-1 /-/-indole (300 mg) in EtOAc (15 ml_) and water (15 ml_) was added iron (465 mg) followed by ammonium chloride (356 mg). The reaction mixture was heated to 60 °C and stirred for 4 hr. The reaction mixture was filtered through Celite and extracted with EtOAc (20 ml_). The EtOAc extract was dried over sodium sulfate, filtered and concentrated under reduced pressure to afford the title compound (200 mg). GCMS m/z 150.1 (M+).
References:
WO2017/153952,2017,A1 Location in patent:Page/Page column 154

926028-83-3
4 suppliers
inquiry

926028-84-4
34 suppliers
$45.00/10mg
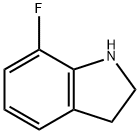
769966-04-3
58 suppliers
inquiry

926028-84-4
34 suppliers
$45.00/10mg
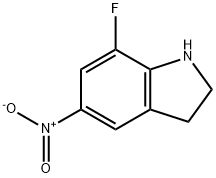
1268816-57-4
14 suppliers
inquiry

926028-84-4
34 suppliers
$45.00/10mg
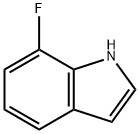
387-44-0
310 suppliers
$24.00/1g

926028-84-4
34 suppliers
$45.00/10mg